Oncolytic activity of reovirus in HPV positive and negative head and neck squamous cell carcinoma
- PMID: 25890191
- PMCID: PMC4348167
- DOI: 10.1186/s40463-015-0062-x
Oncolytic activity of reovirus in HPV positive and negative head and neck squamous cell carcinoma
Abstract
Background: The management of patients with advanced stages of head and neck cancer requires a multidisciplinary and multimodality treatment approach which includes a combination of surgery, radiation, and chemotherapy. These toxic treatment protocols have significantly improved survival outcomes in a distinct population of human papillomavirus (HPV) associated oropharyngeal cancer. HPV negative head and neck squamous cell carcinoma (HNSCC) remains a challenge to treat because there is only a modest improvement in survival with the present treatment regimens, requiring innovative and new treatment approaches. Oncolytic viruses used as low toxicity adjunct cancer therapies are novel, potentially effective treatments for HNSCC. One such oncolytic virus is Respiratory Orphan Enteric virus or reovirus. Susceptibility of HNSCC cells towards reovirus infection and reovirus-induced cell death has been previously demonstrated but has not been compared in HPV positive and negative HNSCC cell lines.
Objectives: To compare the infectivity and oncolytic activity of reovirus in HPV positive and negative HNSCC cell lines.
Methods: Seven HNSCC cell lines were infected with serial dilutions of reovirus. Two cell lines (UM-SCC-47 and UM-SCC-104) were positive for type 16 HPV. Infectivity was measured using a cell-based ELISA assay 18 h after infection. Oncolytic activity was determined using an alamar blue viability assay 96 h after infection. Non-linear regression models were used to calculate the amounts of virus required to infect and to cause cell death in 50% of a given cell line (EC50). EC50 values were compared.
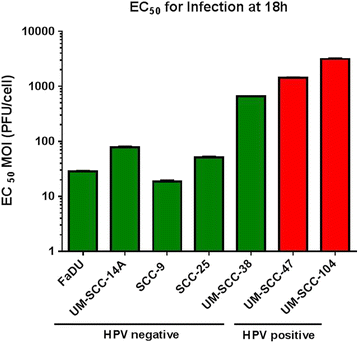
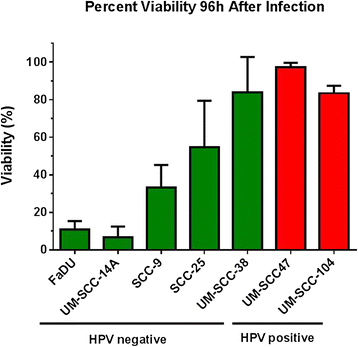
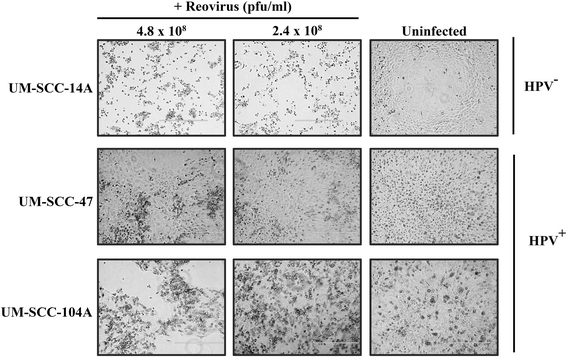
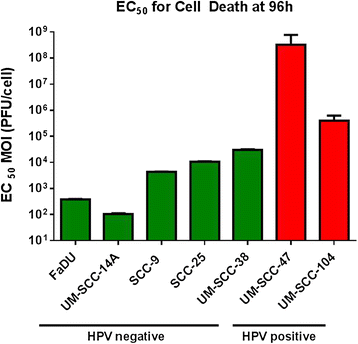
Results: HPV negative cells were more susceptible to viral infection and oncolysis compared to HPV positive cell lines. EC50 for infectivity at 18 h ranged from multiplicity of infection (MOI) values (PFU/cell) of 18.6 (SCC-9) to 3133 (UM-SCC 104). EC50 for cell death at 96 h ranged from a MOI (PFU/cell) of 1.02×10(2) (UM-SCC-14A) to 3.19×10(8) (UM-SCC-47). There was a 3×10(6) fold difference between the least susceptible cell line (UM-SCC-47) and the most susceptible line (UM-SCC 14A) EC50 for cell death at 96 h.
Conclusions: HPV negative HNSCC cell lines appear to demonstrate greater reovirus infectivity and virus-mediated oncolysis compared to HPV positive HNSCC. Reovirus shows promise as a novel therapy in HNSCC, and may be of particular benefit in HPV negative patients.
Figures
Similar articles
-
Characteristics of Human Papillomavirus-Associated Head and Neck Cancers in a Veteran Population.JAMA Otolaryngol Head Neck Surg. 2015 Sep;141(9):790-6. doi: 10.1001/jamaoto.2015.1447. JAMA Otolaryngol Head Neck Surg. 2015. PMID: 26270931
-
Sensitivity of cervical carcinoma cells to vesicular stomatitis virus-induced oncolysis: potential role of human papilloma virus infection.Int J Cancer. 2012 Aug 1;131(3):E204-15. doi: 10.1002/ijc.27404. Epub 2012 Jan 11. Int J Cancer. 2012. PMID: 22173567
-
Chemotherapeutic alteration of VEGF-/PDGF- and PDGF-Rα/β expression by imatinib in HPV-transformed squamous cell carcinoma compared to HPV-negative HNSCC in vitro.Oncol Rep. 2011 Nov;26(5):1099-109. doi: 10.3892/or.2011.1403. Epub 2011 Jul 26. Oncol Rep. 2011. PMID: 21805039
-
What is the best treatment for patients with human papillomavirus-positive and -negative oropharyngeal cancer?Cancer. 2014 May 15;120(10):1462-70. doi: 10.1002/cncr.28595. Epub 2014 Feb 27. Cancer. 2014. PMID: 24578320 Review.
-
Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis.Int J Cancer. 2007 Oct 15;121(8):1813-20. doi: 10.1002/ijc.22851. Int J Cancer. 2007. PMID: 17546592 Review.
Cited by
-
The clinicopathological significances and biological functions of parafibromin expression in head and neck squamous cell carcinomas.Tumour Biol. 2015 Dec;36(12):9487-97. doi: 10.1007/s13277-015-3618-5. Epub 2015 Jul 1. Tumour Biol. 2015. PMID: 26124004
-
Investigation of EZH2 pathways for novel epigenetic treatment strategies in oropharyngeal cancer.J Otolaryngol Head Neck Surg. 2016 Oct 28;45(1):54. doi: 10.1186/s40463-016-0168-9. J Otolaryngol Head Neck Surg. 2016. PMID: 27793210 Free PMC article.
-
Human papillomavirus insertions identify the PIM family of serine/threonine kinases as targetable driver genes in head and neck squamous cell carcinoma.Cancer Lett. 2020 Apr 28;476:23-33. doi: 10.1016/j.canlet.2020.01.012. Epub 2020 Jan 17. Cancer Lett. 2020. PMID: 31958486 Free PMC article.
-
The virome of HPV-positive tonsil squamous cell carcinoma and neck metastasis.Oncotarget. 2020 Jan 21;11(3):282-293. doi: 10.18632/oncotarget.27436. eCollection 2020 Jan 21. Oncotarget. 2020. PMID: 32076488 Free PMC article.
-
Relative Biological Effectiveness of Carbon Ions for Head-and-Neck Squamous Cell Carcinomas According to Human Papillomavirus Status.J Pers Med. 2020 Jul 25;10(3):71. doi: 10.3390/jpm10030071. J Pers Med. 2020. PMID: 32722522 Free PMC article.
References
-
- Rutten H, Pop LA, Janssens GO, Takes RP, Knuijt S, Rooijakkers AF, et al. Long-term outcome and morbidity after treatment with accelerated radiotherapy and weekly cisplatin for locally advanced head-and-neck cancer: Results of a multidisciplinary late morbidity clinic. Int J Radiat Oncol, Biol, Phys. 2011;81(4):923–9. doi: 10.1016/j.ijrobp.2010.07.013. - DOI - PubMed
-
- Laco J, Nekvindova J, Novakova V, Celakovsky P, Dolezalova H, Tucek L, et al. Biologic importance and prognostic significance of selected clinicopathological parameters in patients with oral and oropharyngeal squamous cell carcinoma, with emphasis on smoking, protein p16(INK4a) expression, and HPV status. Neoplasma. 2012;59(4):398–408. doi: 10.4149/neo_2012_052. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

