Lipopolysaccharide preconditioning of adipose-derived stem cells improves liver-regenerating activity of the secretome
- PMID: 25890074
- PMCID: PMC4416308
- DOI: 10.1186/s13287-015-0072-7
Lipopolysaccharide preconditioning of adipose-derived stem cells improves liver-regenerating activity of the secretome
Abstract
Introduction: Growing recognition of paracrine mechanisms in stem cell plasticity has resulted in considerable interest in stem cell-derived secretome. The aim of this study was to investigate the effects of lipopolysaccharide (LPS) preconditioning on the composition and hepatic regenerative activity of adipose-derived stem cell (ASC) secretome.
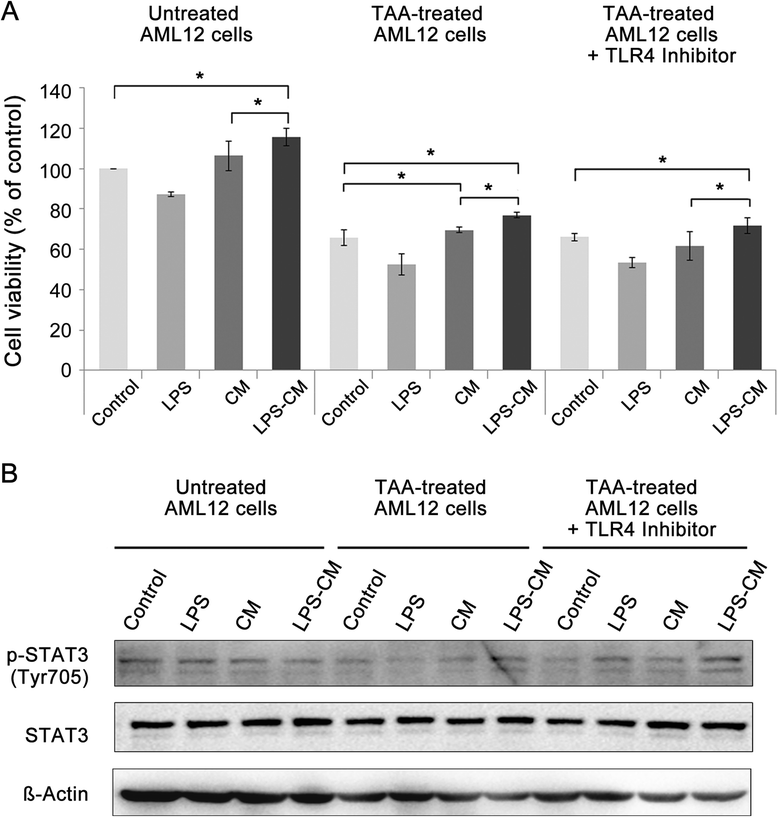
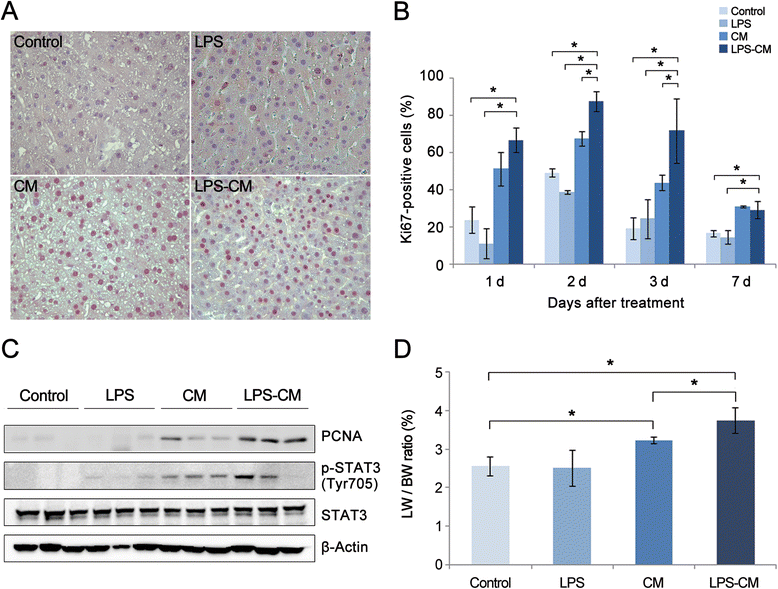
Methods: Conditioned medium (CM) and LPS-CM were obtained after culturing human ASCs without or with low-dose LPS (0.5 ng/mL) for 24 hours. Untreated and thioacetamide-treated mouse AML12 hepatocytes were incubated for 24 hours with the control medium, LPS (0.5 ng/mL), CM, and LPS-CM and then cell viabilities were compared. CM and LPS-CM were also intravenously administered to partially hepatectomized mice, and their effects on liver regeneration were assessed by using liver weight measurements, immunohistochemistry, and Western blotting.
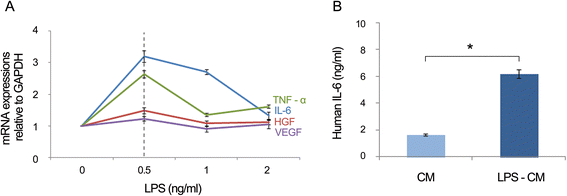
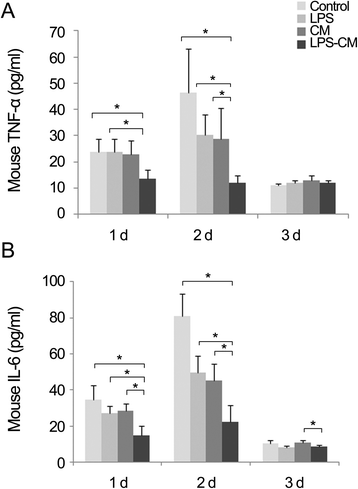
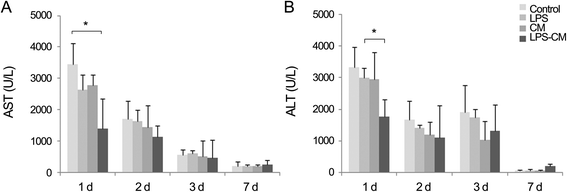
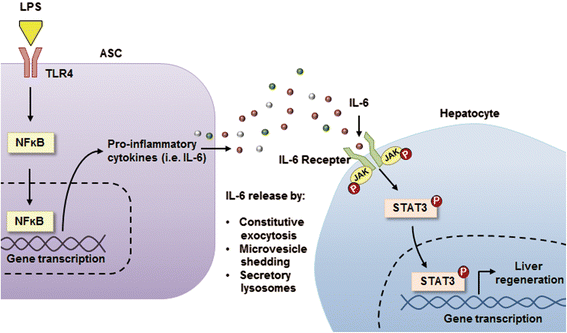
Results: In the in vitro experiments, LPS preconditioning of ASCs enhanced the mRNA expression levels of interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), hepatocyte growth factor, and vascular endothelial growth factor, which evoke inflammatory response or liver regeneration. LPS-CM significantly promoted thioacetamide-damaged AML12 cell viability compared with CM-incubated cells and the control cells (77%, 69%, and 65% P<0.05). In the in vivo experiment, LPS-CM infusion into the partially hepatectomized mice significantly reduced serum IL-6 and TNF-α levels compared with the other groups (P<0.05) on days 1 and 2 after partial hepatectomy. Moreover, LPS-CM infusion enhanced liver regeneration (based on the liver weight changes at day 7 after partial hepatectomy, 3.73% versus 3.22% in the CM group; P<0.05) and significantly reduced the elevated serum levels of aspartate transaminase and alanine transaminase (at day 1, P<0.05).
Conclusions: Our results suggest that LPS preconditioning effectively stimulates ASCs to produce the secretome beneficial to hepatic regeneration. Thus, optimizing ASC secretome profile by LPS preconditioning could be a promising approach to treat liver diseases by using stem cells.
Figures
Similar articles
-
Hypoxic Conditioned Medium From Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration Through JAK/STAT3 Signaling.Stem Cells Transl Med. 2016 Jun;5(6):816-25. doi: 10.5966/sctm.2015-0191. Epub 2016 Apr 21. Stem Cells Transl Med. 2016. PMID: 27102647 Free PMC article.
-
A novel cell-free strategy for promoting mouse liver regeneration: utilization of a conditioned medium from adipose-derived stem cells.Hepatol Int. 2015 Apr;9(2):310-20. doi: 10.1007/s12072-014-9599-4. Epub 2014 Dec 25. Hepatol Int. 2015. PMID: 25788187
-
Determination of optimized oxygen partial pressure to maximize the liver regenerative potential of the secretome obtained from adipose-derived stem cells.Stem Cell Res Ther. 2017 Aug 3;8(1):181. doi: 10.1186/s13287-017-0635-x. Stem Cell Res Ther. 2017. PMID: 28774345 Free PMC article.
-
Review of the adipose derived stem cell secretome.Biochimie. 2013 Dec;95(12):2222-8. doi: 10.1016/j.biochi.2013.06.001. Epub 2013 Jun 14. Biochimie. 2013. PMID: 23770442 Review.
-
Potential application of adipose-derived stem cells and their secretory factors to skin: discussion from both clinical and industrial viewpoints.Expert Opin Biol Ther. 2010 Apr;10(4):495-503. doi: 10.1517/14712591003610598. Expert Opin Biol Ther. 2010. PMID: 20218919 Review.
Cited by
-
How to enhance MSCs therapeutic properties? An insight on potentiation methods.Stem Cell Res Ther. 2024 Sep 27;15(1):331. doi: 10.1186/s13287-024-03935-6. Stem Cell Res Ther. 2024. PMID: 39334487 Free PMC article. Review.
-
Exosomes from preconditioned mesenchymal stem cells: Tissue repair and regeneration.Regen Ther. 2024 Feb 14;25:355-366. doi: 10.1016/j.reth.2024.01.009. eCollection 2024 Mar. Regen Ther. 2024. PMID: 38374989 Free PMC article. Review.
-
Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model.Stem Cell Res Ther. 2021 Jul 28;12(1):427. doi: 10.1186/s13287-021-02507-2. Stem Cell Res Ther. 2021. PMID: 34321073 Free PMC article.
-
New therapeutic approaches of mesenchymal stem cells-derived exosomes.J Biomed Sci. 2021 May 25;28(1):39. doi: 10.1186/s12929-021-00736-4. J Biomed Sci. 2021. PMID: 34030679 Free PMC article. Review.
-
Enhanced Therapeutic Potential of the Secretome Released from Adipose-Derived Stem Cells by PGC-1α-Driven Upregulation of Mitochondrial Proliferation.Int J Mol Sci. 2019 Nov 8;20(22):5589. doi: 10.3390/ijms20225589. Int J Mol Sci. 2019. PMID: 31717375 Free PMC article.
References
-
- Yang Z, Di Santo S, Kalka C. Current developments in the use of stem cell for therapeutic neovascularisation: is the future therapy “cell-free”? Swiss Med Wkly. 2010;140:w13130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

