Mesenchymal stromal cell delivery of full-length tumor necrosis factor-related apoptosis-inducing ligand is superior to soluble type for cancer therapy
- PMID: 25888191
- PMCID: PMC4503823
- DOI: 10.1016/j.jcyt.2015.03.603
Mesenchymal stromal cell delivery of full-length tumor necrosis factor-related apoptosis-inducing ligand is superior to soluble type for cancer therapy
Abstract
Background aims: Mesenchymal stromal cell (MSC) delivery of pro-apoptotic tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is an attractive strategy for anticancer therapy. MSCs expressing full-length human TRAIL (flT) or its soluble form (sT) have previously been shown to be effective for cancer killing. However, a comparison between the two forms has never been performed, leaving it unclear which approach is most effective. This study addresses the issue for the possible clinical application of TRAIL-expressing MSCs in the future.
Methods: MSCs were transduced with lentiviruses expressing flT or an isoleucine zipper-fused sT. TRAIL expression was examined and cancer cell apoptosis was measured after treatment with transduced MSCs or with MSC-derived soluble TRAIL.
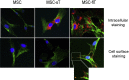
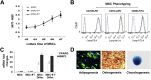
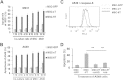
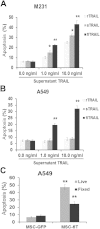
Results: The transduction does not adversely affect cell phenotype. The sT-transduced MSCs (MSC-sT) secrete abundant levels of soluble TRAIL but do not present the protein on the cell surface. Interestingly, the flT-transduced MSCs (MSC-flT) not only express cell-surface TRAIL but also release flT into medium. These cells were examined for inducing apoptosis in 20 cancer cell lines. MSC-sT cells showed very limited effects. By contrast, MSC-flT cells demonstrated high cancer cell-killing efficiency. More importantly, MSC-flT cells can overcome some cancer cell resistance to recombinant TRAIL. In addition, both cell surface flT and secreted flT are functional for inducing apoptosis. The secreted flT was found to have higher cancer cell-killing capacity than either recombinant TRAIL or MSC-secreted sT.
Conclusions: These observations demonstrate that MSC delivery of flT is superior to MSC delivery of sT for cancer therapy.
Keywords: TRAIL; apoptosis; cancer; mesenchymal stromal cell; tumor.
Copyright © 2015 International Society for Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Impact of soluble tumor necrosis factor-related apoptosis-inducing ligand released by engineered adipose mesenchymal stromal cells on white blood cells.Cytotherapy. 2023 Jun;25(6):605-614. doi: 10.1016/j.jcyt.2023.02.008. Epub 2023 Apr 1. Cytotherapy. 2023. PMID: 37012089
-
Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells.Neurosurgery. 2009 Sep;65(3):610-24; discussion 624. doi: 10.1227/01.NEU.0000350227.61132.A7. Neurosurgery. 2009. PMID: 19687708
-
Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy.Cancer Res. 2010 May 1;70(9):3718-29. doi: 10.1158/0008-5472.CAN-09-1865. Epub 2010 Apr 13. Cancer Res. 2010. PMID: 20388793
-
Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour.Int J Mol Sci. 2018 Jul 27;19(8):2188. doi: 10.3390/ijms19082188. Int J Mol Sci. 2018. PMID: 30060445 Free PMC article. Review.
-
TRAIL based therapy: overview of mesenchymal stem cell based delivery and miRNA controlled expression of TRAIL.Asian Pac J Cancer Prev. 2014;15(16):6495-7. doi: 10.7314/apjcp.2014.15.16.6495. Asian Pac J Cancer Prev. 2014. PMID: 25169476 Review.
Cited by
-
EV-T synergizes with AZD5582 to overcome TRAIL resistance through concomitant suppression of cFLIP, MCL-1, and IAPs in hepatocarcinoma.J Mol Med (Berl). 2022 Apr;100(4):629-643. doi: 10.1007/s00109-022-02180-9. Epub 2022 Mar 5. J Mol Med (Berl). 2022. PMID: 35247069
-
A combined antitumor strategy of separately transduced mesenchymal stem cells with soluble TRAIL and IFNβ produces a synergistic activity in the reduction of lymphoma and mice survival enlargement.Mol Med Rep. 2022 Jun;25(6):206. doi: 10.3892/mmr.2022.12722. Epub 2022 Apr 29. Mol Med Rep. 2022. PMID: 35485288 Free PMC article.
-
Engineered human mesenchymal stem cells for neuroblastoma therapeutics.Oncol Rep. 2019 Jul;42(1):35-42. doi: 10.3892/or.2019.7152. Epub 2019 May 8. Oncol Rep. 2019. PMID: 31115546 Free PMC article.
-
Lung delivery of MSCs expressing anti-cancer protein TRAIL visualised with 89Zr-oxine PET-CT.Stem Cell Res Ther. 2020 Jun 26;11(1):256. doi: 10.1186/s13287-020-01770-z. Stem Cell Res Ther. 2020. PMID: 32586403 Free PMC article.
-
The Role of Mesenchymal Stem Cells and Exosomes in Tumor Development and Targeted Antitumor Therapies.Stem Cells Int. 2023 Feb 14;2023:7059289. doi: 10.1155/2023/7059289. eCollection 2023. Stem Cells Int. 2023. PMID: 36824409 Free PMC article. Review.
References
-
- Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.P., Nicholl J.K. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. - PubMed
-
- Pitti R.M., Marsters S.A., Ruppert S., Donahue C.J., Moore A., Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. - PubMed
-
- Walczak H., Miller R.E., Ariail K., Gliniak B., Griffith T.S., Kubin M. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

