An extracytoplasmic function sigma factor-dependent periplasmic glutathione peroxidase is involved in oxidative stress response of Shewanella oneidensis
- PMID: 25887418
- PMCID: PMC4336711
- DOI: 10.1186/s12866-015-0357-0
An extracytoplasmic function sigma factor-dependent periplasmic glutathione peroxidase is involved in oxidative stress response of Shewanella oneidensis
Abstract
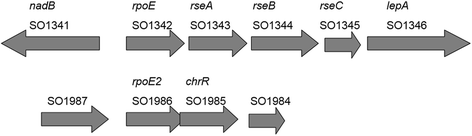
Background: Bacteria use alternative sigma factors (σs) to regulate condition-specific gene expression for survival and Shewanella harbors multiple ECF (extracytoplasmic function) σ genes and cognate anti-sigma factor genes. Here we comparatively analyzed two of the rpoE-like operons in the strain MR-1: rpoE-rseA-rseB-rseC and rpoE2-chrR.
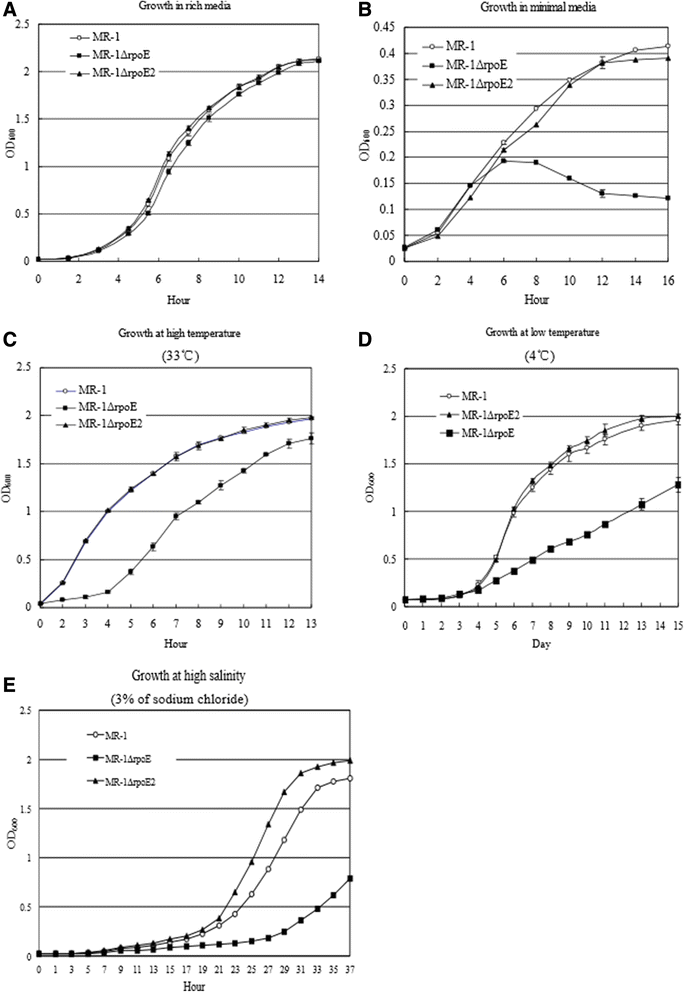
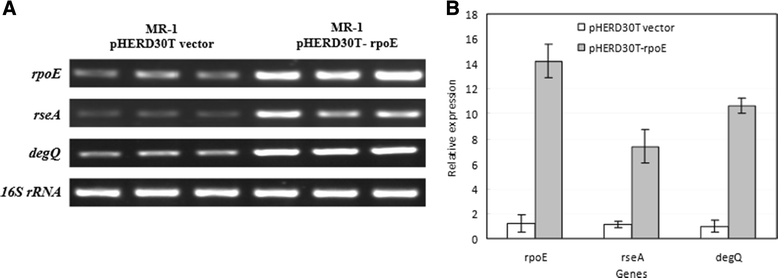
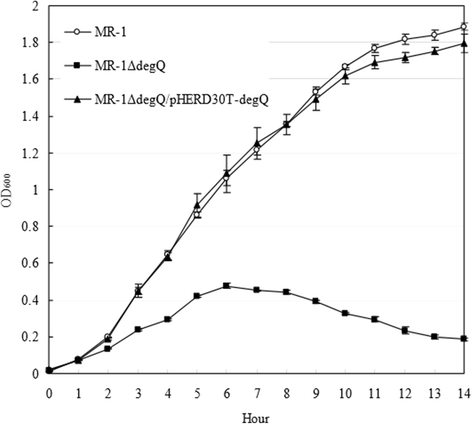
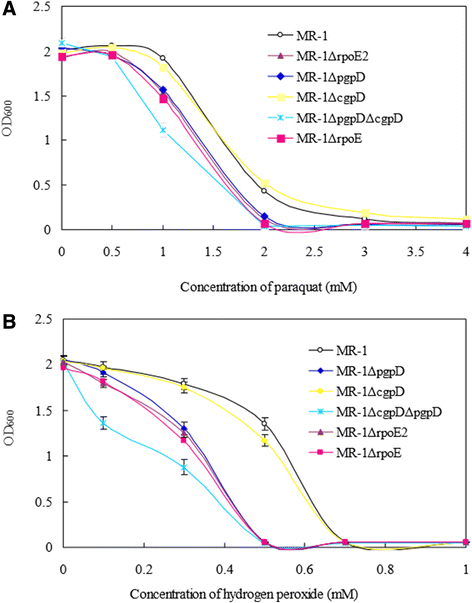
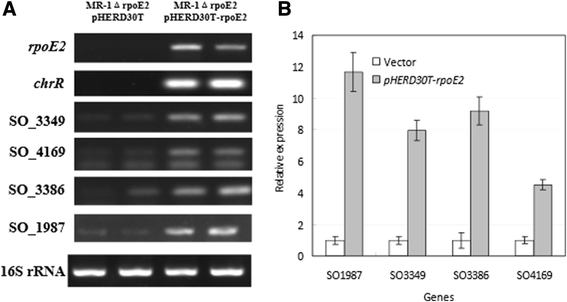
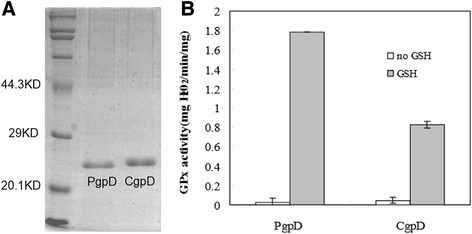
Results: RpoE was important for bacterial growth at low and high temperatures, in the minimal medium, and high salinity. The degP/htrA orthologue, required for growth of Escherichia coli and Pseudomonas aeruginosa at high temperature, is absent in Shewanella, while the degQ gene is RpoE-regulated and is required for bacterial growth at high temperature. RpoE2 was essential for the optimal growth in oxidative stress conditions because the rpoE2 mutant was sensitive to hydrogen peroxide and paraquat. The operon encoding a ferrochelatase paralogue (HemH2) and a periplasmic glutathione peroxidase (PgpD) was identified as RpoE2-dependent. PgpD exhibited higher activities and played a more important role in the oxidative stress responses than the cytoplasmic glutathione peroxidase CgpD under tested conditions. The rpoE2-chrR operon and the identified regulon genes, including pgpD and hemH2, are coincidently absent in several psychrophilic and/or deep-sea Shewanella strains.
Conclusion: In S. oneidensis MR-1, the RpoE-dependent degQ gene is required for optimal growth under high temperature. The rpoE2 and RpoE2-dependent pgpD gene encoding a periplasmic glutathione peroxidase are involved in oxidative stress responses. But rpoE2 is not required for bacterial growth at low temperature and it even affected bacterial growth under salt stress, indicating that there is a tradeoff between the salt resistance and RpoE2-mediated oxidative stress responses.
Figures
Similar articles
-
Differential Regulation of the Two Ferrochelatase Paralogues in Shewanella loihica PV-4 in Response to Environmental Stresses.Appl Environ Microbiol. 2016 Aug 15;82(17):5077-88. doi: 10.1128/AEM.00203-16. Print 2016 Sep 1. Appl Environ Microbiol. 2016. PMID: 27287322 Free PMC article.
-
Differential gene content and gene expression for bacterial evolution and speciation of Shewanella in terms of biosynthesis of heme and heme-requiring proteins.BMC Microbiol. 2019 Jul 30;19(1):173. doi: 10.1186/s12866-019-1549-9. BMC Microbiol. 2019. PMID: 31362704 Free PMC article.
-
A constitutively expressed pair of rpoE2-chrR2 in Azospirillum brasilense Sp7 is required for survival under antibiotic and oxidative stress.Microbiology (Reading). 2013 Feb;159(Pt 2):205-218. doi: 10.1099/mic.0.061937-0. Epub 2012 Oct 11. Microbiology (Reading). 2013. PMID: 23059974
-
Singlet oxygen stress in microorganisms.Adv Microb Physiol. 2011;58:141-73. doi: 10.1016/B978-0-12-381043-4.00004-0. Adv Microb Physiol. 2011. PMID: 21722793 Review.
-
Diversity of extracytoplasmic function sigma (σECF ) factor-dependent signaling in Pseudomonas.Mol Microbiol. 2019 Aug;112(2):356-373. doi: 10.1111/mmi.14331. Epub 2019 Jul 3. Mol Microbiol. 2019. PMID: 31206859 Review.
Cited by
-
Differential Regulation of the Two Ferrochelatase Paralogues in Shewanella loihica PV-4 in Response to Environmental Stresses.Appl Environ Microbiol. 2016 Aug 15;82(17):5077-88. doi: 10.1128/AEM.00203-16. Print 2016 Sep 1. Appl Environ Microbiol. 2016. PMID: 27287322 Free PMC article.
-
The Interaction between Oxidative Stress Biomarkers and Gut Microbiota in the Antioxidant Effects of Extracts from Sonchus brachyotus DC. in Oxazolone-Induced Intestinal Oxidative Stress in Adult Zebrafish.Antioxidants (Basel). 2023 Jan 13;12(1):192. doi: 10.3390/antiox12010192. Antioxidants (Basel). 2023. PMID: 36671053 Free PMC article.
-
Transcriptome Analysis Reveals Cr(VI) Adaptation Mechanisms in Klebsiella sp. Strain AqSCr.Front Microbiol. 2021 May 27;12:656589. doi: 10.3389/fmicb.2021.656589. eCollection 2021. Front Microbiol. 2021. PMID: 34122372 Free PMC article.
-
RpoN (σ54) Is Required for Floc Formation but Not for Extracellular Polysaccharide Biosynthesis in a Floc-Forming Aquincola tertiaricarbonis Strain.Appl Environ Microbiol. 2017 Jun 30;83(14):e00709-17. doi: 10.1128/AEM.00709-17. Print 2017 Jul 15. Appl Environ Microbiol. 2017. PMID: 28500044 Free PMC article.
-
Physiological and Proteomic Responses of Dairy Buffalo to Heat Stress Induced by Different Altitudes.Metabolites. 2022 Sep 27;12(10):909. doi: 10.3390/metabo12100909. Metabolites. 2022. PMID: 36295811 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

