Megakaryoblastic leukemia-1 is required for the development of bleomycin-induced pulmonary fibrosis
- PMID: 25885656
- PMCID: PMC4392778
- DOI: 10.1186/s12931-015-0206-6
Megakaryoblastic leukemia-1 is required for the development of bleomycin-induced pulmonary fibrosis
Abstract
Background: Fibrosing disorders of the lung, such as idiopathic pulmonary fibrosis, are characterized by progressive extracellular matrix accumulation that is driven by myofibroblasts. The transcription factor megakaryoblastic leukemia-1 (MKL1) mediates myofibroblast differentiation in response to several profibrotic stimuli, but the role it plays in mediating pulmonary fibrosis has not been fully elucidated. In this study, we utilized mice that had a germline deletion of MKL1 (MKL1 (-,-)) to determine the role that MKL1 plays in the development of bleomycin-induced pulmonary fibrosis.
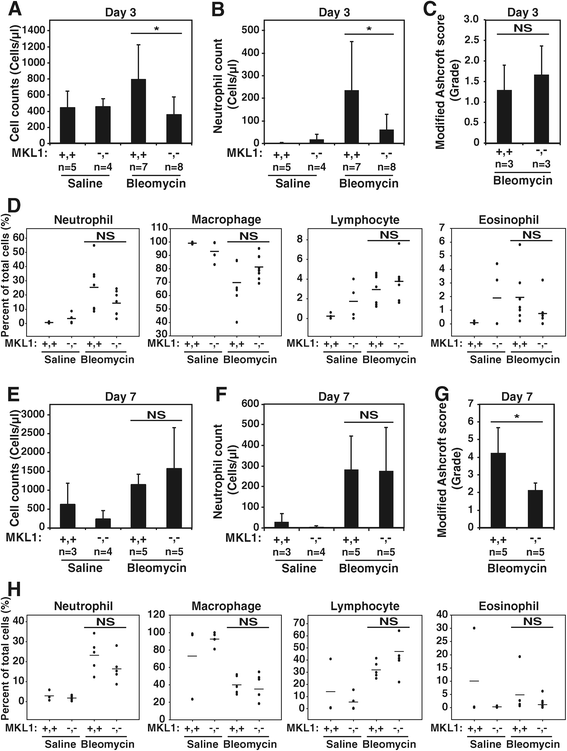
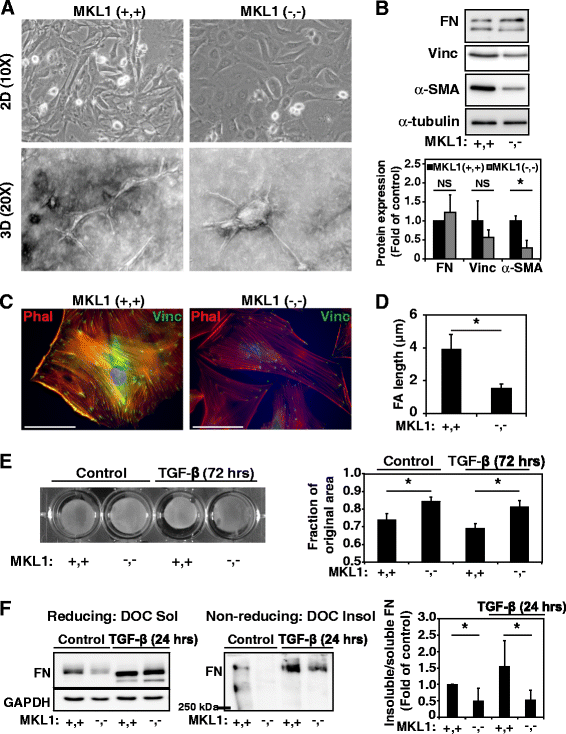
Methods: Bleomycin or normal saline were intratracheally delivered to 9 to 12 week old female MKL1 (+,+) and MKL1 (-,-) mice. Mice were assessed for weight loss and survival to 28 days. Inflammatory responses were assessed through bronchoalveolar lavage at days 3 and 7 post-treatment. The development of pulmonary fibrosis was characterized using hydroxyproline assay and histological staining. MKL1 (+,+) and MKL1 (-,-) mouse lung fibroblasts were isolated to compare morphologic, gene expression and functional differences.
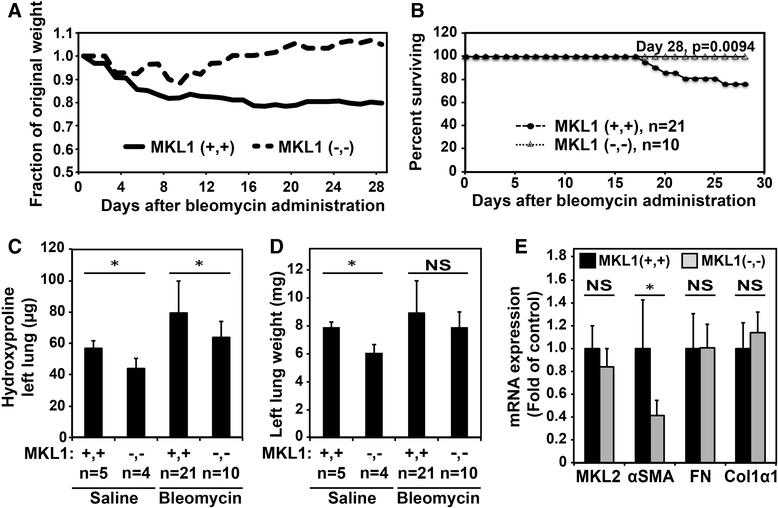
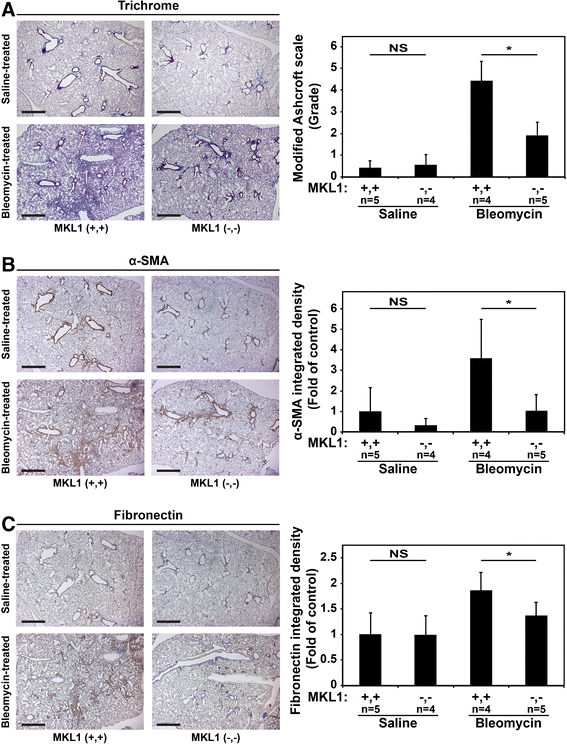
Results: MKL1 (-,-) mice demonstrated increased survival, attenuated weight loss, and decreased collagen accumulation compared to wild-type animals 28-days after intratracheal instillation of bleomycin. Histological analysis demonstrated decreased trichrome, smooth muscle α-actin, and fibronectin staining in MKL1(-,-) mice compared to MKL1 (+,+) controls. Differential cell counts from bronchoalveolar lavage demonstrated that there was attenuated neutrophilia 3 days after bleomycin administration, but no difference at day 7. Isolated mouse lung fibroblasts from MKL1 (-,-) mice had decreased contractility and deposited less fibronectin matrix compared to wild-type controls, suggesting a defect in key remodeling functions.
Conclusions: Altogether, these data demonstrate that MKL1 plays a significant role in mediating the fibrotic response to bleomycin injury. Loss of MKL1 attenuated early neutrophil influx, as well as myofibroblast-mediated remodeling. Targeting MKL1 activity may therefore be a useful strategy in treating pulmonary fibrosis.
Figures
Similar articles
-
Fibroblast growth factor 2 decreases bleomycin-induced pulmonary fibrosis and inhibits fibroblast collagen production and myofibroblast differentiation.J Pathol. 2018 Sep;246(1):54-66. doi: 10.1002/path.5106. Epub 2018 Jul 5. J Pathol. 2018. PMID: 29873400 Free PMC article.
-
Cthrc1 lowers pulmonary collagen associated with bleomycin-induced fibrosis and protects lung function.Physiol Rep. 2017 Mar;5(5):e13115. doi: 10.14814/phy2.13115. Physiol Rep. 2017. PMID: 28292882 Free PMC article.
-
Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice.Am J Physiol Lung Cell Mol Physiol. 2002 Mar;282(3):L585-93. doi: 10.1152/ajplung.00151.2001. Am J Physiol Lung Cell Mol Physiol. 2002. PMID: 11839555
-
Hepatocyte growth factor in lung repair and pulmonary fibrosis.Acta Pharmacol Sin. 2011 Jan;32(1):12-20. doi: 10.1038/aps.2010.90. Epub 2010 Dec 6. Acta Pharmacol Sin. 2011. PMID: 21131996 Free PMC article. Review.
-
Is there a role for specialized pro-resolving mediators in pulmonary fibrosis?Pharmacol Ther. 2023 Jul;247:108460. doi: 10.1016/j.pharmthera.2023.108460. Epub 2023 May 26. Pharmacol Ther. 2023. PMID: 37244406 Free PMC article. Review.
Cited by
-
Expression of serum response factor in the lung mesenchyme is essential for development of pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2021 Jul 1;321(1):L174-L188. doi: 10.1152/ajplung.00323.2020. Epub 2021 May 12. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33978489 Free PMC article.
-
MKL1-induced lncRNA SNHG18 drives the growth and metastasis of non-small cell lung cancer via the miR-211-5p/BRD4 axis.Cell Death Dis. 2021 Jan 26;12(1):128. doi: 10.1038/s41419-021-03399-z. Cell Death Dis. 2021. PMID: 33500406 Free PMC article.
-
Resident Fibroblast MKL1 Is Sufficient to Drive Pro-fibrogenic Response in Mice.Front Cell Dev Biol. 2022 Feb 1;9:812748. doi: 10.3389/fcell.2021.812748. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35178401 Free PMC article.
-
Myofibroblast-specific inhibition of the Rho kinase-MRTF-SRF pathway using nanotechnology for the prevention of pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2023 Feb 1;324(2):L190-L198. doi: 10.1152/ajplung.00086.2022. Epub 2023 Jan 10. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 36625494 Free PMC article.
-
[64Cu]Cu-PEG-FUD peptide for noninvasive and sensitive detection of murine pulmonary fibrosis.Sci Adv. 2024 Apr 12;10(15):eadj1444. doi: 10.1126/sciadv.adj1444. Epub 2024 Apr 10. Sci Adv. 2024. PMID: 38598637 Free PMC article.
References
-
- Phan SH. Fibroblast phenotypes in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29(3 Suppl):S87–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

