Synthesis and pharmacophore modelling of 2,6,9-trisubstituted purine derivatives and their potential role as apoptosis-inducing agents in cancer cell lines
- PMID: 25884555
- PMCID: PMC6272238
- DOI: 10.3390/molecules20046808
Synthesis and pharmacophore modelling of 2,6,9-trisubstituted purine derivatives and their potential role as apoptosis-inducing agents in cancer cell lines
Abstract
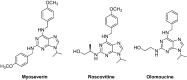
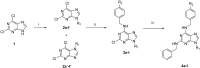
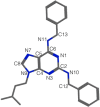
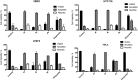
A series of 2,6,9-trisubstituted purine derivatives have been synthesized and investigated for their potential role as antitumor agents. Twelve compounds were obtained by a three step synthetic procedure using microwave irradiation in a pivotal step. All compounds were evaluated in vitro to determine their potential effect on cell toxicity by the MTT method and flow cytometry analysis on four cancer cells lines and Vero cells. Three out of twelve compounds were found to be promising agents compared to a known and effective anticancer drug, etoposide, in three out of four cancer cell lines assayed with considerable selectivity. Preliminary flow cytometry data suggests that compounds mentioned above induce apoptosis on these cells. The main structural requirements for their activity for each cancer cell line were characterized with a preliminary pharmacophore model, which identified aromatic centers, hydrogen acceptor/donor center and a hydrophobic area. These features were consistent with the cytotoxic activity of the assayed compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
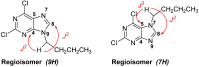
Similar articles
-
Promising 2,6,9-Trisubstituted Purine Derivatives for Anticancer Compounds: Synthesis, 3D-QSAR, and Preliminary Biological Assays.Int J Mol Sci. 2019 Dec 25;21(1):161. doi: 10.3390/ijms21010161. Int J Mol Sci. 2019. PMID: 31881717 Free PMC article.
-
Synthesis and in vitro biological evaluation of 2,6,9-trisubstituted purines targeting multiple cyclin-dependent kinases.Eur J Med Chem. 2013 Mar;61:61-72. doi: 10.1016/j.ejmech.2012.06.036. Epub 2012 Jun 23. Eur J Med Chem. 2013. PMID: 22770608
-
Selective anti-tubercular purines: synthesis and chemotherapeutic properties of 6-aryl- and 6-heteroaryl-9-benzylpurines.Bioorg Med Chem. 2005 Dec 1;13(23):6360-73. doi: 10.1016/j.bmc.2005.06.054. Epub 2005 Aug 2. Bioorg Med Chem. 2005. PMID: 16081292
-
Terpenyl-purines from the sea.Mar Drugs. 2009 Dec 23;7(4):833-49. doi: 10.3390/md7040833. Mar Drugs. 2009. PMID: 20098613 Free PMC article. Review.
-
Recent Developments and Future Perspectives of Purine Derivatives as a Promising Scaffold in Drug Discovery.Curr Top Med Chem. 2024;24(6):541-579. doi: 10.2174/0115680266290152240110074034. Curr Top Med Chem. 2024. PMID: 38288806 Review.
Cited by
-
Synthesis and fluorescent properties of N(9)-alkylated 2-amino-6-triazolylpurines and 7-deazapurines.Beilstein J Org Chem. 2019 Feb 15;15:474-489. doi: 10.3762/bjoc.15.41. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 30873231 Free PMC article.
-
Discovery of New Sulfonamide Carbonic Anhydrase IX Inhibitors Incorporating Nitrogenous Bases.ACS Med Chem Lett. 2017 Nov 21;8(12):1314-1319. doi: 10.1021/acsmedchemlett.7b00399. eCollection 2017 Dec 14. ACS Med Chem Lett. 2017. PMID: 29259754 Free PMC article.
-
Promising 2,6,9-Trisubstituted Purine Derivatives for Anticancer Compounds: Synthesis, 3D-QSAR, and Preliminary Biological Assays.Int J Mol Sci. 2019 Dec 25;21(1):161. doi: 10.3390/ijms21010161. Int J Mol Sci. 2019. PMID: 31881717 Free PMC article.
-
Design, Synthesis, In Silico Studies and Inhibitory Activity towards Bcr-Abl, BTK and FLT3-ITD of New 2,6,9-Trisubstituted Purine Derivatives as Potential Agents for the Treatment of Leukaemia.Pharmaceutics. 2022 Jun 17;14(6):1294. doi: 10.3390/pharmaceutics14061294. Pharmaceutics. 2022. PMID: 35745866 Free PMC article.
-
Coumarins and Gastrointestinal Cancer: A New Therapeutic Option?Front Oncol. 2021 Oct 11;11:752784. doi: 10.3389/fonc.2021.752784. eCollection 2021. Front Oncol. 2021. PMID: 34707995 Free PMC article. Review.
References
-
- Rosemeyer H. The Chemodiversity of Purine as a Constituent of Natural Products. Chem. Biodivers. 2004;1:361–401. - PubMed
-
- Legraverend M., Grierson D.S. The purines: Potent and versatile small molecule inhibitors and modulators of key biological targets. Bioorg. Med. Chem. 2006;14:3987–4006. - PubMed
-
- Hansen S.W., Skovsgaard T., Sorensen J.B. Treatment of small cell lung cancer with 6-mercaptopurine: A phase II study. Cancer Treat. Rep. 1985;69:555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

