Hyper-excitability and epilepsy generated by chronic early-life stress
- PMID: 25884016
- PMCID: PMC4395864
- DOI: 10.1016/j.ynstr.2015.03.001
Hyper-excitability and epilepsy generated by chronic early-life stress
Abstract
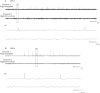
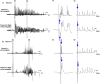
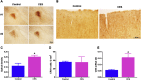
Epilepsy is more prevalent in populations with high measures of stress, but the neurobiological mechanisms are unclear. Stress is a common precipitant of seizures in individuals with epilepsy, and may provoke seizures by several mechanisms including changes in neurotransmitter and hormone levels within the brain. Importantly, stress during sensitive periods early in life contributes to 'brain programming', influencing neuronal function and brain networks. However, it is unclear if early-life stress influences limbic excitability and promotes epilepsy. Here we used an established, naturalistic model of chronic early-life stress (CES), and employed chronic cortical and limbic video-EEGs combined with molecular and cellular techniques to probe the contributions of stress to age-specific epilepsies and network hyperexcitability and identify the underlying mechanisms. In control male rats, EEGs obtained throughout development were normal and no seizures were observed. EEGs demonstrated epileptic spikes and spike series in the majority of rats experiencing CES, and 57% of CES rats developed seizures: Behavioral events resembling the human age-specific epilepsy infantile spasms occurred in 11/23 (48%), accompanied by EEG spikes and/or electrodecrements, and two additional rats (9%) developed limbic seizures that involved the amygdala. Probing for stress-dependent, endogenous convulsant molecules within amygdala, we examined the expression of the pro-convulsant neuropeptide corticotropin-releasing hormone (CRH), and found a significant increase of amygdalar--but not cortical--CRH expression in adolescent CES rats. In conclusion, CES of limited duration has long-lasting effects on brain excitability and may promote age-specific seizures and epilepsy. Whereas the mechanisms involved require further study, these findings provide important insights into environmental contributions to early-life seizures.
Keywords: amygdala; corticotropin releasing hormone; epilepsy; infantile spasms; seizures; stress.
Figures

Similar articles
-
Anhedonia Following Early-Life Adversity Involves Aberrant Interaction of Reward and Anxiety Circuits and Is Reversed by Partial Silencing of Amygdala Corticotropin-Releasing Hormone Gene.Biol Psychiatry. 2018 Jan 15;83(2):137-147. doi: 10.1016/j.biopsych.2017.08.023. Epub 2017 Sep 7. Biol Psychiatry. 2018. PMID: 29033027 Free PMC article.
-
Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis.Brain. 2006 Apr;129(Pt 4):911-22. doi: 10.1093/brain/awl018. Epub 2006 Jan 30. Brain. 2006. PMID: 16446281 Free PMC article.
-
Basic mechanisms of catastrophic epilepsy -- overview from animal models.Brain Dev. 2013 Sep;35(8):748-56. doi: 10.1016/j.braindev.2012.12.005. Epub 2013 Jan 11. Brain Dev. 2013. PMID: 23312951 Free PMC article. Review.
-
ACTH does not control neonatal seizures induced by administration of exogenous corticotropin-releasing hormone.Epilepsia. 1995 Feb;36(2):174-8. doi: 10.1111/j.1528-1157.1995.tb00977.x. Epilepsia. 1995. PMID: 7821275 Free PMC article.
-
Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain.Trends Neurosci. 1998 Nov;21(11):471-6. doi: 10.1016/s0166-2236(98)01275-2. Trends Neurosci. 1998. PMID: 9829688 Free PMC article. Review.
Cited by
-
Developmental and epileptic encephalopathies: from genetic heterogeneity to phenotypic continuum.Physiol Rev. 2023 Jan 1;103(1):433-513. doi: 10.1152/physrev.00063.2021. Epub 2022 Aug 11. Physiol Rev. 2023. PMID: 35951482 Free PMC article. Review.
-
Positive and negative early life experiences differentially modulate long term survival and amyloid protein levels in a mouse model of Alzheimer's disease.Oncotarget. 2016 Jun 28;7(26):39118-39135. doi: 10.18632/oncotarget.9776. Oncotarget. 2016. PMID: 27259247 Free PMC article.
-
Cortical Parvalbumin-Positive Interneuron Development and Function Are Altered in the APC Conditional Knockout Mouse Model of Infantile and Epileptic Spasms Syndrome.J Neurosci. 2023 Feb 22;43(8):1422-1440. doi: 10.1523/JNEUROSCI.0572-22.2022. Epub 2023 Jan 30. J Neurosci. 2023. PMID: 36717229 Free PMC article.
-
The neurobiology of childhood trauma, from early physical pain onwards: as relevant as ever in today's fractured world.Eur J Psychotraumatol. 2022 Oct 18;13(2):2131969. doi: 10.1080/20008066.2022.2131969. eCollection 2022. Eur J Psychotraumatol. 2022. PMID: 36276555 Free PMC article. Review.
-
Brain Networks Reorganization During Maturation and Healthy Aging-Emphases for Resilience.Front Psychiatry. 2018 Nov 21;9:601. doi: 10.3389/fpsyt.2018.00601. eCollection 2018. Front Psychiatry. 2018. PMID: 30519196 Free PMC article. Review.
References
-
- Aldenhoff J.B., Gruol D.L., Rivier J., Vale W., Siggins G.R. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
