Lin41/Trim71 is essential for mouse development and specifically expressed in postnatal ependymal cells of the brain
- PMID: 25883935
- PMCID: PMC4382986
- DOI: 10.3389/fcell.2015.00020
Lin41/Trim71 is essential for mouse development and specifically expressed in postnatal ependymal cells of the brain
Abstract
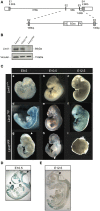
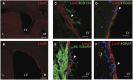
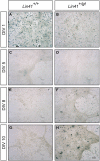
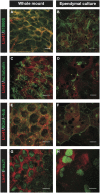
Lin41/Trim71 is a heterochronic gene encoding a member of the Trim-NHL protein family, and is the original, genetically defined target of the microRNA let-7 in C. elegans. Both the LIN41 protein and multiple regulatory microRNA binding sites in the 3' UTR of the mRNA are highly conserved from nematodes to humans. Functional studies have described essential roles for mouse LIN41 in embryonic stem cells, cellular reprogramming and the timing of embryonic neurogenesis. We have used a new gene trap mouse line deficient in Lin41 to characterize Lin41 expression during embryonic development and in the postnatal central nervous system (CNS). In the embryo, Lin41 is required for embryonic viability and neural tube closure. Nevertheless, neurosphere assays suggest that Lin41 is not required for adult neurogenesis. Instead, we show that Lin41 promoter activity and protein expression in the postnatal CNS is restricted to ependymal cells lining the walls of the four ventricles. We use ependymal cell culture to confirm reestablishment of Lin41 expression during differentiation of ependymal progenitors to post-mitotic cells possessing motile cilia. Our results reveal that terminally differentiated ependymal cells express Lin41, a gene to date associated with self-renewing stem cells.
Keywords: Lin41; Trim71; ependyma; gene trap; neural tube closure; neurogenesis.
Figures


Similar articles
-
Repressing Ago2 mRNA translation by Trim71 maintains pluripotency through inhibiting let-7 microRNAs.Elife. 2021 Feb 18;10:e66288. doi: 10.7554/eLife.66288. Elife. 2021. PMID: 33599613 Free PMC article.
-
The ubiquitin ligase LIN41/TRIM71 targets p53 to antagonize cell death and differentiation pathways during stem cell differentiation.Cell Death Differ. 2017 Jun;24(6):1063-1078. doi: 10.1038/cdd.2017.54. Epub 2017 Apr 21. Cell Death Differ. 2017. PMID: 28430184 Free PMC article.
-
LIN41 Post-transcriptionally Silences mRNAs by Two Distinct and Position-Dependent Mechanisms.Mol Cell. 2017 Feb 2;65(3):476-489.e4. doi: 10.1016/j.molcel.2016.12.010. Epub 2017 Jan 19. Mol Cell. 2017. PMID: 28111013
-
LIN-41/TRIM71: emancipation of a miRNA target.Genes Dev. 2013 Mar 15;27(6):581-9. doi: 10.1101/gad.207266.112. Genes Dev. 2013. PMID: 23512656 Free PMC article. Review.
-
Molecular and biological functions of TRIM-NHL RNA-binding proteins.Wiley Interdiscip Rev RNA. 2021 Mar;12(2):e1620. doi: 10.1002/wrna.1620. Epub 2020 Aug 1. Wiley Interdiscip Rev RNA. 2021. PMID: 32738036 Free PMC article. Review.
Cited by
-
De Novo Mutation in Genes Regulating Neural Stem Cell Fate in Human Congenital Hydrocephalus.Neuron. 2018 Jul 25;99(2):302-314.e4. doi: 10.1016/j.neuron.2018.06.019. Epub 2018 Jul 5. Neuron. 2018. PMID: 29983323 Free PMC article.
-
Repressing Ago2 mRNA translation by Trim71 maintains pluripotency through inhibiting let-7 microRNAs.Elife. 2021 Feb 18;10:e66288. doi: 10.7554/eLife.66288. Elife. 2021. PMID: 33599613 Free PMC article.
-
Gain-of-function mutations in Trim71 linked to congenital hydrocephalus.PLoS Biol. 2023 Feb 9;21(2):e3001993. doi: 10.1371/journal.pbio.3001993. eCollection 2023 Feb. PLoS Biol. 2023. PMID: 36757939 Free PMC article.
-
Multiple Mechanisms Inactivate the LIN-41 RNA-Binding Protein To Ensure a Robust Oocyte-to-Embryo Transition in Caenorhabditis elegans.Genetics. 2018 Nov;210(3):1011-1037. doi: 10.1534/genetics.118.301421. Epub 2018 Sep 11. Genetics. 2018. PMID: 30206186 Free PMC article.
-
Different congenital hydrocephalus-associated mutations in Trim71 impair stem cell differentiation via distinct gain-of-function mechanisms.PLoS Biol. 2023 Feb 9;21(2):e3001947. doi: 10.1371/journal.pbio.3001947. eCollection 2023 Feb. PLoS Biol. 2023. PMID: 36757932 Free PMC article.
References
-
- Abeliovich A., Gerber D., Tanaka O., Katsuki M., Graybiel A. M., Tonegawa S. (1992). On somatic recombination in the central nervous system of transgenic mice. Science 257, 404–410. - PubMed
-
- Calaora V., Chazal G., Nielsen P. J., Rougon G., Moreau H. (1996). mCD24 expression in the developing mouse brain and in zones of secondary neurogenesis in the adult. Neuroscience 73, 581–594. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

