Selective loss of glucocerebrosidase activity in sporadic Parkinson's disease and dementia with Lewy bodies
- PMID: 25881142
- PMCID: PMC4428238
- DOI: 10.1186/s13024-015-0010-2
Selective loss of glucocerebrosidase activity in sporadic Parkinson's disease and dementia with Lewy bodies
Abstract
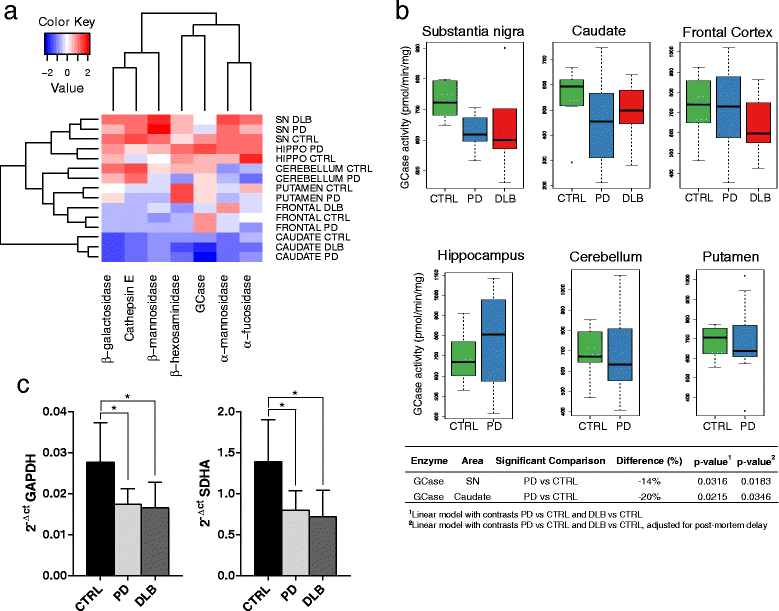
Background: Lysosomal dysfunction is thought to be a prominent feature in the pathogenetic events leading to Parkinson's disease (PD). This view is supported by the evidence that mutations in GBA gene, coding the lysosomal hydrolase β-glucocerebrosidase (GCase), are a common genetic risk factor for PD. Recently, GCase activity has been shown to be decreased in substantia nigra and in cerebrospinal fluid of patients diagnosed with PD or dementia with Lewy Bodies (DLB). Here we measured the activity of GCase and other endo-lysosomal enzymes in different brain regions (frontal cortex, caudate, hippocampus, substantia nigra, cerebellum) from PD (n = 26), DLB (n = 16) and age-matched control (n = 13) subjects, screened for GBA mutations. The relative changes in GCase gene expression in substantia nigra were also quantified by real-time PCR. The role of potential confounders (age, sex and post-mortem delay) was also determined.
Findings: Substantia nigra showed a high activity level for almost all the lysosomal enzymes assessed. GCase activity was significantly decreased in the caudate (-23%) and substantia nigra (-12%) of the PD group; the same trend was observed in DLB. In both groups, a decrease in GCase mRNA was documented in substantia nigra. No other lysosomal hydrolase defects were determined.
Conclusion: The high level of lysosomal enzymes activity observed in substantia nigra, together with the selective reduction of GCase in PD and DLB patients, further support the link between lysosomal dysfunction and PD pathogenesis, favoring the possible role of GCase as biomarker of synucleinopathy. Mapping the lysosomal enzyme activities across different brain areas can further contribute to the understanding of the role of lysosomal derangement in PD and other synucleinopathies.
Figures
Similar articles
-
Characterization of Brain Lysosomal Activities in GBA-Related and Sporadic Parkinson's Disease and Dementia with Lewy Bodies.Mol Neurobiol. 2019 Feb;56(2):1344-1355. doi: 10.1007/s12035-018-1090-0. Epub 2018 Jun 8. Mol Neurobiol. 2019. PMID: 29948939 Free PMC article.
-
Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains.Ann Neurol. 2012 Sep;72(3):455-63. doi: 10.1002/ana.23614. Ann Neurol. 2012. PMID: 23034917 Free PMC article.
-
Path mediation analysis reveals GBA impacts Lewy body disease status by increasing α-synuclein levels.Neurobiol Dis. 2019 Jan;121:205-213. doi: 10.1016/j.nbd.2018.09.015. Epub 2018 Sep 17. Neurobiol Dis. 2019. PMID: 30236861
-
Glucocerebrosidase and Parkinson disease: Recent advances.Mol Cell Neurosci. 2015 May;66(Pt A):37-42. doi: 10.1016/j.mcn.2015.03.013. Epub 2015 Mar 20. Mol Cell Neurosci. 2015. PMID: 25802027 Free PMC article. Review.
-
[GBA mutations and Parkinson's disease].Sheng Li Xue Bao. 2018 Jun 25;70(3):294-300. Sheng Li Xue Bao. 2018. PMID: 29926071 Review. Chinese.
Cited by
-
Association of CSF Glucocerebrosidase Activity With the Risk of Incident Dementia in Patients With Parkinson Disease.Neurology. 2023 Jan 24;100(4):e388-e395. doi: 10.1212/WNL.0000000000201418. Epub 2022 Oct 17. Neurology. 2023. PMID: 36253102 Free PMC article.
-
Gaucher disease provides a unique window into Parkinson disease pathogenesis.Nat Rev Neurol. 2024 Sep;20(9):526-540. doi: 10.1038/s41582-024-00999-z. Epub 2024 Aug 6. Nat Rev Neurol. 2024. PMID: 39107435 Review.
-
Inhibition of cysteine protease cathepsin L increases the level and activity of lysosomal glucocerebrosidase.JCI Insight. 2024 Feb 8;9(3):e169594. doi: 10.1172/jci.insight.169594. JCI Insight. 2024. PMID: 38329128 Free PMC article.
-
Towards physiologically relevant human pluripotent stem cell (hPSC) models of Parkinson's disease.Stem Cell Res Ther. 2021 Apr 29;12(1):253. doi: 10.1186/s13287-021-02326-5. Stem Cell Res Ther. 2021. PMID: 33926571 Free PMC article. Review.
-
A new glucocerebrosidase-deficient neuronal cell model provides a tool to probe pathophysiology and therapeutics for Gaucher disease.Dis Model Mech. 2016 Jul 1;9(7):769-78. doi: 10.1242/dmm.024588. Epub 2016 May 19. Dis Model Mech. 2016. PMID: 27482815 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

