Graves' disease is associated with a defective expression of the immune regulatory molecule galectin-9 in antigen-presenting dendritic cells
- PMID: 25880730
- PMCID: PMC4399981
- DOI: 10.1371/journal.pone.0123938
Graves' disease is associated with a defective expression of the immune regulatory molecule galectin-9 in antigen-presenting dendritic cells
Abstract
Introduction: Patients with autoimmune thyroid disease (AITD) show defects in their immune-regulatory mechanisms. Herein we assessed the expression and function of galectin-1 and galectin-9 (Gal-1, Gal-9) in dendritic cells (DCs) from patients with AITD.
Materials and methods: Peripheral blood samples from 25 patients with Graves' disease (GD), 11 Hashimoto's thyroiditis (HT), and 24 healthy subjects were studied. Thyroid tissue samples from 44 patients with AITD and 22 patients with goiter were also analyzed. Expression and function of Gal-1 and Gal-9 was assessed by quantitative RT-PCR, immunofluorescence and flow cytometry.
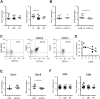
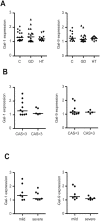
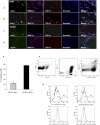
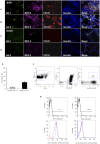
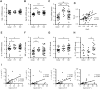
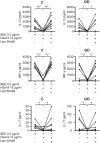
Results: A diminished expression of Gal-9, but not of Gal-1, by peripheral blood DCs was observed in GD patients, mainly in those with Graves´ ophthalmopathy, and a significant negative association between disease severity and Gal-9 expression was detected. In addition, the mRNA levels of Gal-9 and its ligand TIM-3 were increased in thyroid tissue from AITD patients and its expression was associated with the levels of Th1/Th12/Th17 cytokines. Immunofluorescence studies proved that intrathyroidal Gal-9 expression was confined to DCs and macrophages. Finally, in vitro functional assays showed that exogenous Gal-9 had a suppressive effect on the release of Th1/Th2/Th17 cytokines by DC/lymphocyte autologous co-cultures from both AITD patients and healthy controls.
Conclusions: The altered pattern of expression of Gal-9 in peripheral blood DCs from GD patients, its correlation with disease severity as well as its ability to suppress cytokine release suggest that Gal-9 could be involved in the pathogenesis of AITD.
Conflict of interest statement
Figures
Similar articles
-
Plasmacytoid dendritic cells in patients with autoimmune thyroid disease.J Clin Endocrinol Metab. 2013 Jul;98(7):2822-33. doi: 10.1210/jc.2013-1273. Epub 2013 May 10. J Clin Endocrinol Metab. 2013. PMID: 23666960
-
Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and in the proportion of Th17 cells in intractable Graves' disease.Thyroid. 2009 May;19(5):495-501. doi: 10.1089/thy.2008.0423. Thyroid. 2009. PMID: 19415997
-
Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto's thyroiditis (Th1) and Graves' disease (Th2).Neuroimmunomodulation. 2004;11(4):209-13. doi: 10.1159/000078438. Neuroimmunomodulation. 2004. PMID: 15249726
-
Regulatory B and T cell responses in patients with autoimmune thyroid disease and healthy controls.Dan Med J. 2016 Feb;63(2):B5177. Dan Med J. 2016. PMID: 26836805 Review.
-
The pathogenesis of thyroid autoimmune diseases: New T lymphocytes - Cytokines circuits beyond the Th1-Th2 paradigm.J Cell Physiol. 2019 Mar;234(3):2204-2216. doi: 10.1002/jcp.27180. Epub 2018 Sep 24. J Cell Physiol. 2019. PMID: 30246383 Review.
Cited by
-
Genetic Selection of Peptide Aptamers That Interact and Inhibit Both Small Protein B and Alternative Ribosome-Rescue Factor A of Aeromonas veronii C4.Front Microbiol. 2016 Aug 18;7:1228. doi: 10.3389/fmicb.2016.01228. eCollection 2016. Front Microbiol. 2016. PMID: 27588015 Free PMC article.
-
Translational Implication of Galectin-9 in the Pathogenesis and Treatment of Viral Infection.Int J Mol Sci. 2017 Oct 8;18(10):2108. doi: 10.3390/ijms18102108. Int J Mol Sci. 2017. PMID: 28991189 Free PMC article. Review.
-
Decreased Expression of TIM-3 on Th17 Cells Associated with Ophthalmopathy in Patients with Graves' Disease.Curr Mol Med. 2018;18(2):83-90. doi: 10.2174/1566524018666180705105753. Curr Mol Med. 2018. PMID: 29974826 Free PMC article.
-
miRNA-5119 regulates immune checkpoints in dendritic cells to enhance breast cancer immunotherapy.Cancer Immunol Immunother. 2020 Jun;69(6):951-967. doi: 10.1007/s00262-020-02507-w. Epub 2020 Feb 20. Cancer Immunol Immunother. 2020. PMID: 32076794 Free PMC article.
-
Immunomodulatory Factors Galectin-9 and Interferon-Gamma Synergize to Induce Expression of Rate-Limiting Enzymes of the Kynurenine Pathway in the Mouse Hippocampus.Front Immunol. 2016 Oct 17;7:422. doi: 10.3389/fimmu.2016.00422. eCollection 2016. Front Immunol. 2016. PMID: 27799931 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

