The presence of nuclear cactus in the early Drosophila embryo may extend the dynamic range of the dorsal gradient
- PMID: 25879657
- PMCID: PMC4400154
- DOI: 10.1371/journal.pcbi.1004159
The presence of nuclear cactus in the early Drosophila embryo may extend the dynamic range of the dorsal gradient
Abstract
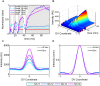
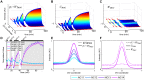
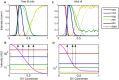
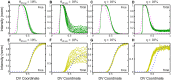
In a developing embryo, the spatial distribution of a signaling molecule, or a morphogen gradient, has been hypothesized to carry positional information to pattern tissues. Recent measurements of morphogen distribution have allowed us to subject this hypothesis to rigorous physical testing. In the early Drosophila embryo, measurements of the morphogen Dorsal, which is a transcription factor responsible for initiating the earliest zygotic patterns along the dorsal-ventral axis, have revealed a gradient that is too narrow to pattern the entire axis. In this study, we use a mathematical model of Dorsal dynamics, fit to experimental data, to determine the ability of the Dorsal gradient to regulate gene expression across the entire dorsal-ventral axis. We found that two assumptions are required for the model to match experimental data in both Dorsal distribution and gene expression patterns. First, we assume that Cactus, an inhibitor that binds to Dorsal and prevents it from entering the nuclei, must itself be present in the nuclei. And second, we assume that fluorescence measurements of Dorsal reflect both free Dorsal and Cactus-bound Dorsal. Our model explains the dynamic behavior of the Dorsal gradient at lateral and dorsal positions of the embryo, the ability of Dorsal to regulate gene expression across the entire dorsal-ventral axis, and the robustness of gene expression to stochastic effects. Our results have a general implication for interpreting fluorescence-based measurements of signaling molecules.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
A novel function for the IκB inhibitor Cactus in promoting Dorsal nuclear localization and activity in the Drosophila embryo.Development. 2017 Aug 15;144(16):2907-2913. doi: 10.1242/dev.145557. Epub 2017 Jul 13. Development. 2017. PMID: 28705899 Free PMC article.
-
Robustness of the Dorsal morphogen gradient with respect to morphogen dosage.PLoS Comput Biol. 2020 Apr 6;16(4):e1007750. doi: 10.1371/journal.pcbi.1007750. eCollection 2020 Apr. PLoS Comput Biol. 2020. PMID: 32251432 Free PMC article.
-
cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos.Development. 1991 Jun;112(2):371-88. doi: 10.1242/dev.112.2.371. Development. 1991. PMID: 1794309
-
Dorsal ventral polarity and pattern formation in the Drosophila embryo.Semin Cell Biol. 1990 Jun;1(3):137-49. Semin Cell Biol. 1990. PMID: 2103885 Review.
-
Formation, interpretation, and regulation of the Drosophila Dorsal/NF-κB gradient.Curr Top Dev Biol. 2020;137:143-191. doi: 10.1016/bs.ctdb.2019.11.007. Epub 2019 Dec 26. Curr Top Dev Biol. 2020. PMID: 32143742 Review.
Cited by
-
A novel function for the IκB inhibitor Cactus in promoting Dorsal nuclear localization and activity in the Drosophila embryo.Development. 2017 Aug 15;144(16):2907-2913. doi: 10.1242/dev.145557. Epub 2017 Jul 13. Development. 2017. PMID: 28705899 Free PMC article.
-
ISRES+: an improved evolutionary strategy for function minimization to estimate the free parameters of systems biology models.Bioinformatics. 2023 Jul 1;39(7):btad403. doi: 10.1093/bioinformatics/btad403. Bioinformatics. 2023. PMID: 37354523 Free PMC article.
-
A reaction-diffusion network model predicts a dual role of Cactus/IκB to regulate Dorsal/NFκB nuclear translocation in Drosophila.PLoS Comput Biol. 2021 May 27;17(5):e1009040. doi: 10.1371/journal.pcbi.1009040. eCollection 2021 May. PLoS Comput Biol. 2021. PMID: 34043616 Free PMC article.
-
Identifying effective evolutionary strategies-based protocol for uncovering reaction kinetic parameters under the effect of measurement noises.BMC Biol. 2024 Oct 14;22(1):235. doi: 10.1186/s12915-024-02019-4. BMC Biol. 2024. PMID: 39402553 Free PMC article.
-
Mechanism and implications of morphogen shuttling: Lessons learned from dorsal and Cactus in Drosophila.Dev Biol. 2020 May 1;461(1):13-18. doi: 10.1016/j.ydbio.2020.01.011. Epub 2020 Jan 24. Dev Biol. 2020. PMID: 31987808 Free PMC article.
References
-
- Roth S, Stein D, Nüsslein-Volhard C. A Gradient of Nuclear Localization of the dorsal Protein Determines Dorsoventral Pattern in the Drosophila Embryo. Cell. 1989;59:1189–1202. - PubMed
-
- Roth S, Hiromi Y, Godt D, Nüsslein-Volhard C. cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos. Dev. 1991. June;112(2):371–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

