The quantal component of synaptic transmission from sensory hair cells to the vestibular calyx
- PMID: 25878150
- PMCID: PMC4473518
- DOI: 10.1152/jn.00055.2015
The quantal component of synaptic transmission from sensory hair cells to the vestibular calyx
Abstract
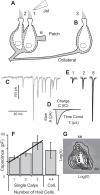
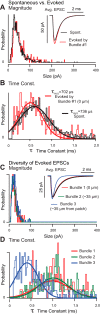
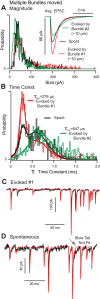
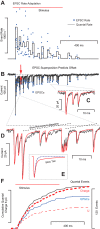
Spontaneous and stimulus-evoked excitatory postsynaptic currents (EPSCs) were recorded in calyx nerve terminals from the turtle vestibular lagena to quantify key attributes of quantal transmission at this synapse. On average, EPSC events had a magnitude of ∼ 42 pA, a rise time constant of τ(0) ∼ 229 μs, decayed to baseline with a time constant of τ(R) ∼ 690 μs, and carried ∼ 46 fC of charge. Individual EPSCs varied in magnitude and decay time constant. Variability in the EPSC decay time constant was hair cell dependent and due in part to a slow protraction of the EPSC in some cases. Variability in EPSC size was well described by an integer summation of unitary quanta, with each quanta of glutamate gating a unitary postsynaptic current of ∼ 23 pA. The unitary charge was ∼ 26 fC for EPSCs with a simple exponential decay and increased to ∼ 48 fC for EPSCs exhibiting a slow protraction. The EPSC magnitude and the number of simultaneous unitary quanta within each event increased with presynaptic stimulus intensity. During tonic hair cell depolarization, both the EPSC magnitude and event rate exhibited adaptive run down over time. Present data from a reptilian calyx are remarkably similar to noncalyceal vestibular synaptic terminals in diverse species, indicating that the skewed EPSC size distribution and multiquantal release might be an ancestral property of inner ear ribbon synapses.
Keywords: auditory; synapse; unitary quanta; vestibular.
Copyright © 2015 the American Physiological Society.
Figures
Similar articles
-
The unitary event underlying multiquantal EPSCs at a hair cell's ribbon synapse.J Neurosci. 2009 Jun 10;29(23):7558-68. doi: 10.1523/JNEUROSCI.0514-09.2009. J Neurosci. 2009. PMID: 19515924 Free PMC article.
-
AMPA receptor-mediated rapid EPSCs in vestibular calyx afferents.J Neurophysiol. 2017 Jun 1;117(6):2312-2323. doi: 10.1152/jn.00394.2016. Epub 2017 Mar 15. J Neurophysiol. 2017. PMID: 28298303 Free PMC article.
-
Locus of frequency-dependent depression identified with multiple-probability fluctuation analysis at rat climbing fibre-Purkinje cell synapses.J Physiol. 1998 Aug 1;510 ( Pt 3)(Pt 3):881-902. doi: 10.1111/j.1469-7793.1998.881bj.x. J Physiol. 1998. PMID: 9660900 Free PMC article.
-
Quantal analysis of excitatory postsynaptic currents at the hippocampal mossy fiber-CA3 pyramidal cell synapse.Adv Second Messenger Phosphoprotein Res. 1994;29:235-60. doi: 10.1016/s1040-7952(06)80019-4. Adv Second Messenger Phosphoprotein Res. 1994. PMID: 7848714 Review.
-
The afferent synapse of cochlear hair cells.Curr Opin Neurobiol. 2003 Aug;13(4):452-8. doi: 10.1016/s0959-4388(03)00098-9. Curr Opin Neurobiol. 2003. PMID: 12965293 Review.
Cited by
-
Semicircular canal biomechanics in health and disease.J Neurophysiol. 2019 Mar 1;121(3):732-755. doi: 10.1152/jn.00708.2018. Epub 2018 Dec 19. J Neurophysiol. 2019. PMID: 30565972 Free PMC article. Review.
-
Models of utricular bouton afferents: role of afferent-hair cell connectivity in determining spike train regularity.J Neurophysiol. 2017 May 1;117(5):1969-1986. doi: 10.1152/jn.00895.2016. Epub 2017 Feb 15. J Neurophysiol. 2017. PMID: 28202575 Free PMC article.
-
Age-dependent structural reorganization of utricular ribbon synapses.Front Cell Dev Biol. 2023 Aug 10;11:1178992. doi: 10.3389/fcell.2023.1178992. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37635868 Free PMC article.
-
A mathematical model for mechanical activation and compound action potential generation by the utricle in response to sound and vibration.Front Neurol. 2023 Mar 27;14:1109506. doi: 10.3389/fneur.2023.1109506. eCollection 2023. Front Neurol. 2023. PMID: 37051057 Free PMC article.
-
Inhibition of Ionic Currents by Fluoxetine in Vestibular Calyces in Different Epithelial Loci.Int J Mol Sci. 2024 Aug 13;25(16):8801. doi: 10.3390/ijms25168801. Int J Mol Sci. 2024. PMID: 39201487 Free PMC article.
References
-
- Chapochnikov NM, Takago H, Huang CH, Pangrsic T, Khimich D, Neef J, Auge E, Gottfert F, Hell SW, Wichmann C, Wolf F, Moser T. Uniquantal release through a dynamic fusion pore is a candidate mechanism of hair cell exocytosis. Neuron 83: 1389–1403, 2014. - PubMed
-
- Contini D, Zampini V, Tavazzani E, Magistretti J, Russo G, Prigioni I, Masetto S. Intercellular K(+) accumulation depolarizes type I vestibular hair cells and their associated afferent nerve calyx. Neuroscience 227: 232–246, 2012. - PubMed
-
- Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res 175: 256–267, 2006. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

