Bariatric surgery in morbidly obese insulin resistant humans normalises insulin signalling but not insulin-stimulated glucose disposal
- PMID: 25876175
- PMCID: PMC4395354
- DOI: 10.1371/journal.pone.0120084
Bariatric surgery in morbidly obese insulin resistant humans normalises insulin signalling but not insulin-stimulated glucose disposal
Abstract
Aims: Weight-loss after bariatric surgery improves insulin sensitivity, but the underlying molecular mechanism is not clear. To ascertain the effect of bariatric surgery on insulin signalling, we examined glucose disposal and Akt activation in morbidly obese volunteers before and after Roux-en-Y gastric bypass surgery (RYGB), and compared this to lean volunteers.
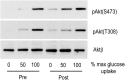
Materials and methods: The hyperinsulinaemic euglycaemic clamp, at five infusion rates, was used to determine glucose disposal rates (GDR) in eight morbidly obese (body mass index, BMI=47.3 ± 2.2 kg/m(2)) patients, before and after RYGB, and in eight lean volunteers (BMI=20.7 ± 0.7 kg/m2). Biopsies of brachioradialis muscle, taken at fasting and insulin concentrations that induced half-maximal (GDR50) and maximal (GDR100) GDR in each subject, were used to examine the phosphorylation of Akt-Thr308, Akt-473, and pras40, in vivo biomarkers for Akt activity.
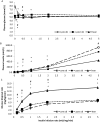
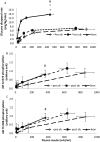
Results: Pre-operatively, insulin-stimulated GDR was lower in the obese compared to the lean individuals (P<0.001). Weight-loss of 29.9 ± 4 kg after surgery significantly improved GDR50 (P=0.004) but not GDR100 (P=0.3). These subjects still remained significantly more insulin resistant than the lean individuals (p<0.001). Weight loss increased insulin-stimulated skeletal muscle Akt-Thr308 and Akt-Ser473 phosphorylation, P=0.02 and P=0.03 respectively (MANCOVA), and Akt activity towards the substrate PRAS40 (P=0.003, MANCOVA), and in contrast to GDR, were fully normalised after the surgery (obese vs lean, P=0.6, P=0.35, P=0.46, respectively).
Conclusions: Our data show that although Akt activity substantially improved after surgery, it did not lead to a full restoration of insulin-stimulated glucose disposal. This suggests that a major defect downstream of, or parallel to, Akt signalling remains after significant weight-loss.
Conflict of interest statement
Figures
Similar articles
-
Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients.J Hepatol. 2014 Feb;60(2):377-83. doi: 10.1016/j.jhep.2013.09.012. Epub 2013 Sep 20. J Hepatol. 2014. PMID: 24060855 Clinical Trial.
-
Early effect of Roux-en-Y gastric bypass on insulin sensitivity and signaling.Surg Obes Relat Dis. 2016 Jan;12(1):42-7. doi: 10.1016/j.soard.2015.06.005. Epub 2015 Jun 10. Surg Obes Relat Dis. 2016. PMID: 26483070
-
Insulin resistance in nondiabetic morbidly obese patients: effect of bariatric surgery.Obes Res. 2003 Dec;11(12):1495-501. doi: 10.1038/oby.2003.200. Obes Res. 2003. PMID: 14694214
-
Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass.Dan Med J. 2015 Apr;62(4):B5057. Dan Med J. 2015. PMID: 25872541 Review.
-
Clinical epigenetics and restoring of metabolic health in severely obese patients undergoing batriatric and metabolic surgery.Updates Surg. 2022 Apr;74(2):431-438. doi: 10.1007/s13304-021-01162-9. Epub 2021 Oct 2. Updates Surg. 2022. PMID: 34599748 Free PMC article. Review.
Cited by
-
Bistable insulin response: The win-win solution for glycemic control.iScience. 2022 Nov 13;25(12):105561. doi: 10.1016/j.isci.2022.105561. eCollection 2022 Dec 22. iScience. 2022. PMID: 36465102 Free PMC article.
-
The Feasibility of Examining the Effects of Gastric Bypass Surgery on Intestinal Metabolism: Prospective, Longitudinal Mechanistic Clinical Trial.JMIR Res Protoc. 2019 Jan 24;8(1):e12459. doi: 10.2196/12459. JMIR Res Protoc. 2019. PMID: 30679147 Free PMC article.
-
Whole Egg Consumption Impairs Insulin Sensitivity in a Rat Model of Obesity and Type 2 Diabetes.Curr Dev Nutr. 2019 Mar 11;3(4):nzz015. doi: 10.1093/cdn/nzz015. eCollection 2019 Apr. Curr Dev Nutr. 2019. PMID: 31008440 Free PMC article.
-
Leukocyte Activation Profile Assessed by Raman Spectroscopy Helps Diagnosing Infection and Sepsis.Crit Care Explor. 2021 May 12;3(5):e0394. doi: 10.1097/CCE.0000000000000394. eCollection 2021 May. Crit Care Explor. 2021. PMID: 34079942 Free PMC article.
-
Longer-Term Physiological and Metabolic Effects of Gastric Bypass Surgery.Curr Diab Rep. 2016 Jun;16(6):50. doi: 10.1007/s11892-016-0747-1. Curr Diab Rep. 2016. PMID: 27091444 Free PMC article. Review.
References
-
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight-Gain as a Risk Factor for Clinical Diabetes-Mellitus in Women. Annals of internal medicine. 1995;122(7):481–6. - PubMed
-
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–26. - PubMed
-
- Bonadonna RC, Groop L, Kraemer N, Ferrannini E, Del Prato S, DeFronzo RA. Obesity and insulin resistance in humans: a dose-response study. Metabolism. 1990;39(5):452–9. - PubMed
-
- Watson RT, Pessin JE. Bridging the GAP between insulin signaling and GLUT4 translocation. Trends in Biochemical Sciences. 2006;31(4):215–22. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

