Determinants of vaccine immunogenicity in HIV-infected pregnant women: analysis of B and T cell responses to pandemic H1N1 monovalent vaccine
- PMID: 25874544
- PMCID: PMC4395240
- DOI: 10.1371/journal.pone.0122431
Determinants of vaccine immunogenicity in HIV-infected pregnant women: analysis of B and T cell responses to pandemic H1N1 monovalent vaccine
Abstract
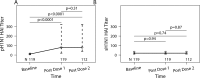
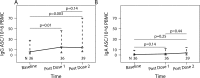
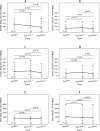
Influenza infections have high frequency and morbidity in HIV-infected pregnant women, underscoring the importance of vaccine-conferred protection. To identify the factors that determine vaccine immunogenicity in this group, we characterized the relationship of B- and T-cell responses to pandemic H1N1 (pH1N1) vaccine with HIV-associated immunologic and virologic characteristics. pH1N1 and seasonal-H1N1 (sH1N1) antibodies were measured in 119 HIV-infected pregnant women after two double-strength pH1N1 vaccine doses. pH1N1-IgG and IgA B-cell FluoroSpot, pH1N1- and sH1N1-interferon γ (IFNγ) and granzyme B (GrB) T-cell FluoroSpot, and flow cytometric characterization of B- and T-cell subsets were performed in 57 subjects. pH1N1-antibodies increased after vaccination, but less than previously described in healthy adults. pH1N1-IgG memory B cells (Bmem) increased, IFNγ-effector T-cells (Teff) decreased, and IgA Bmem and GrB Teff did not change. pH1N1-antibodies and Teff were significantly correlated with each other and with sH1N1-HAI and Teff, respectively, before and after vaccination. pH1N1-antibody responses to the vaccine significantly increased with high proportions of CD4+, low CD8+ and low CD8+HLADR+CD38+ activated (Tact) cells. pH1N1-IgG Bmem responses increased with high proportions of CD19+CD27+CD21- activated B cells (Bact), high CD8+CD39+ regulatory T cells (Treg), and low CD19+CD27-CD21- exhausted B cells (Bexhaust). IFNγ-Teff responses increased with low HIV plasma RNA, CD8+HLADR+CD38+ Tact, CD4+FoxP3+ Treg and CD19+IL10+ Breg. In conclusion, pre-existing antibody and Teff responses to sH1N1 were associated with increased responses to pH1N1 vaccination in HIV-infected pregnant women suggesting an important role for heterosubtypic immunologic memory. High CD4+% T cells were associated with increased, whereas high HIV replication, Tact and Bexhaust were associated with decreased vaccine immunogenicity. High Treg increased antibody responses but decreased Teff responses to the vaccine. The proportions of immature and transitional B cells did not affect the responses to vaccine. Increased Bact were associated with high Bmem responses to the vaccine.
Conflict of interest statement
Figures
Similar articles
-
Vaccination of HIV-infected pregnant women: implications for protection of their young infants.Trop Dis Travel Med Vaccines. 2017 Jan 6;3:1. doi: 10.1186/s40794-016-0044-7. eCollection 2017. Trop Dis Travel Med Vaccines. 2017. PMID: 28883971 Free PMC article. Review.
-
Characterization of functional antibody and memory B-cell responses to pH1N1 monovalent vaccine in HIV-infected children and youth.PLoS One. 2015 Mar 18;10(3):e0118567. doi: 10.1371/journal.pone.0118567. eCollection 2015. PLoS One. 2015. PMID: 25785995 Free PMC article. Clinical Trial.
-
Heterogeneity of T Cell Responses to Pandemic pH1N1 Monovalent Vaccine in HIV-Infected Pregnant Women.AIDS Res Hum Retroviruses. 2015 Nov;31(11):1170-7. doi: 10.1089/aid.2015.0151. Epub 2015 Aug 31. AIDS Res Hum Retroviruses. 2015. PMID: 26322930 Free PMC article.
-
High proportions of regulatory B and T cells are associated with decreased cellular responses to pH1N1 influenza vaccine in HIV-infected children and youth (IMPAACT P1088).Hum Vaccin Immunother. 2013 May;9(5):957-68. doi: 10.4161/hv.23774. Epub 2013 Jan 31. Hum Vaccin Immunother. 2013. PMID: 23370281 Free PMC article.
-
Influenza and HIV: lessons from the 2009 H1N1 influenza pandemic.Curr HIV/AIDS Rep. 2011 Sep;8(3):181-91. doi: 10.1007/s11904-011-0086-4. Curr HIV/AIDS Rep. 2011. PMID: 21710214 Review.
Cited by
-
A FluoroSpot B assay for the detection of IgA and IgG SARS-CoV-2 spike-specific memory B cells: Optimization and qualification for use in COVID-19 vaccine trials.J Immunol Methods. 2023 Apr;515:113457. doi: 10.1016/j.jim.2023.113457. Epub 2023 Mar 11. J Immunol Methods. 2023. PMID: 36914088 Free PMC article.
-
The Role of Cluster of Differentiation 39 (CD39) and Purinergic Signaling Pathway in Viral Infections.Pathogens. 2023 Feb 8;12(2):279. doi: 10.3390/pathogens12020279. Pathogens. 2023. PMID: 36839551 Free PMC article. Review.
-
B and T Cell Phenotypic Profiles of African HIV-Infected and HIV-Exposed Uninfected Infants: Associations with Antibody Responses to the Pentavalent Rotavirus Vaccine.Front Immunol. 2018 Jan 19;8:2002. doi: 10.3389/fimmu.2017.02002. eCollection 2017. Front Immunol. 2018. PMID: 29403482 Free PMC article.
-
Vaccination of HIV-infected pregnant women: implications for protection of their young infants.Trop Dis Travel Med Vaccines. 2017 Jan 6;3:1. doi: 10.1186/s40794-016-0044-7. eCollection 2017. Trop Dis Travel Med Vaccines. 2017. PMID: 28883971 Free PMC article. Review.
-
Survival and predictors of mortality among human immunodeficiency virus patients on anti-retroviral treatment at Jinka Hospital, South Omo, Ethiopia: a six years retrospective cohort study.Epidemiol Health. 2016 Nov 6;38:e2016049. doi: 10.4178/epih.e2016049. eCollection 2016. Epidemiol Health. 2016. PMID: 27820957 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- N01HD33162/HD/NICHD NIH HHS/United States
- HHSN267200800001G/DK/NIDDK NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- N01-DK-9-001/DK/NIDDK NIH HHS/United States
- UL1 TR000154/TR/NCATS NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- U01 AI068616/AI/NIAID NIH HHS/United States
- HHSN267200800001C/HD/NICHD NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- N01 HD033162/HD/NICHD NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- AI068632/AI/NIAID NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- U01 AI41110/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

