Fas/FasL pathway participates in regulation of antiviral and inflammatory response during mousepox infection of lungs
- PMID: 25873756
- PMCID: PMC4385687
- DOI: 10.1155/2015/281613
Fas/FasL pathway participates in regulation of antiviral and inflammatory response during mousepox infection of lungs
Abstract
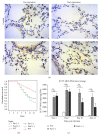
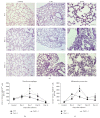
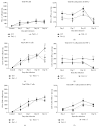
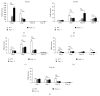
Fas receptor-Fas ligand (FasL) signalling is involved in apoptosis of immune cells as well as of the virus infected target cells but increasing evidence accumulates on Fas as a mediator of apoptosis-independent processes such as induction of activating and proinflammatory signals. In this study, we examined the role of Fas/FasL pathway in inflammatory and antiviral response in lungs using a mousepox model applied to C57BL6/J, B6. MRL-Faslpr/J, and B6Smn.C3-Faslgld/J mice. Ectromelia virus (ECTV) infection of Fas- and FasL-deficient mice led to increased virus titers in lungs and decreased migration of IFN-γ expressing NK cells, CD4+ T cells, CD8+ T cells, and decreased IL-15 expression. The lungs of ECTV-infected Fas- and FasL-deficient mice showed significant inflammation during later phases of infection accompanied by decreased expression of anti-inflammatory IL-10 and TGF-β1 cytokines and disturbances in CXCL1 and CXCL9 expression. Experiments in vitro demonstrated that ECTV-infected cultures of epithelial cells, but not macrophages, upregulate Fas and FasL and are susceptible to Fas-induced apoptosis. Our study demonstrates that Fas/FasL pathway during ECTV infection of the lungs plays an important role in controlling local inflammatory response and mounting of antiviral response.
Figures
Similar articles
-
A lack of Fas/FasL signalling leads to disturbances in the antiviral response during ectromelia virus infection.Arch Virol. 2016 Apr;161(4):913-28. doi: 10.1007/s00705-015-2746-y. Epub 2016 Jan 18. Arch Virol. 2016. PMID: 26780774
-
Role of Fas/FasL signaling in regulation of anti-viral response during HSV-2 vaginal infection in mice.Immunobiology. 2014 Dec;219(12):932-43. doi: 10.1016/j.imbio.2014.07.021. Epub 2014 Aug 5. Immunobiology. 2014. PMID: 25129477
-
Fas/FasL pathway participates in resolution of mucosal inflammatory response early during HSV-2 infection.Immunobiology. 2014 Jan;219(1):64-77. doi: 10.1016/j.imbio.2013.08.002. Epub 2013 Aug 15. Immunobiology. 2014. PMID: 24028839
-
The role of Fas/FasL in the metastatic potential of osteosarcoma and targeting this pathway for the treatment of osteosarcoma lung metastases.Cancer Treat Res. 2009;152:497-508. doi: 10.1007/978-1-4419-0284-9_29. Cancer Treat Res. 2009. PMID: 20213411 Review.
-
Immune privilege or inflammation? The paradoxical effects of Fas ligand.Arch Immunol Ther Exp (Warsz). 2000;48(2):73-9. Arch Immunol Ther Exp (Warsz). 2000. PMID: 10807046 Review.
Cited by
-
MicroRNA-25 Negatively Regulates Cerebral Ischemia/Reperfusion Injury-Induced Cell Apoptosis Through Fas/FasL Pathway.J Mol Neurosci. 2016 Apr;58(4):507-16. doi: 10.1007/s12031-016-0712-0. Epub 2016 Jan 14. J Mol Neurosci. 2016. PMID: 26768135
-
Investigating the possible mechanisms of pirfenidone to be targeted as a promising anti-inflammatory, anti-fibrotic, anti-oxidant, anti-apoptotic, anti-tumor, and/or anti-SARS-CoV-2.Life Sci. 2022 Nov 15;309:121048. doi: 10.1016/j.lfs.2022.121048. Epub 2022 Oct 7. Life Sci. 2022. PMID: 36209833 Free PMC article. Review.
-
The long road to TRAIL therapy: a TRAILshort detour.Oncotarget. 2021 Mar 30;12(7):589-591. doi: 10.18632/oncotarget.27902. eCollection 2021 Mar 30. Oncotarget. 2021. PMID: 33868580 Free PMC article. No abstract available.
-
HBV and HIV/HBV Infected Patients Have Distinct Immune Exhaustion and Apoptotic Serum Biomarker Profiles.Pathog Immun. 2019;4(1):39-65. doi: 10.20411/pai.v4i1.267. Epub 2019 Feb 13. Pathog Immun. 2019. PMID: 30815625 Free PMC article.
-
Matrix metalloproteinase-7 protects against acute kidney injury by priming renal tubules for survival and regeneration.Kidney Int. 2019 May;95(5):1167-1180. doi: 10.1016/j.kint.2018.11.043. Epub 2019 Mar 8. Kidney Int. 2019. PMID: 30878215 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

