Heavy metal complexation of thiol-containing peptides from soy glycinin hydrolysates
- PMID: 25867477
- PMCID: PMC4425066
- DOI: 10.3390/ijms16048040
Heavy metal complexation of thiol-containing peptides from soy glycinin hydrolysates
Abstract
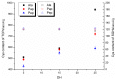
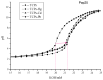
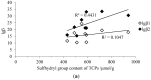
Many thiol-containing molecules show heavy metal complexation ability and are used as antidotes. In this study, the potential function associated with thiol-containing peptides (TCPs) from soy protein hydrolysates as natural detoxicants for heavy metals is reported. TCPs enriched by Thiopropyl-Sepharose 6B covalent chromatography had different molecular weight distributions as well as different numbers of proton dissociable groups, depending on the proteases and degree of hydrolysis. The major contribution of sulfhydryl groups was confirmed by the largest pH decrease between 8.0 and 8.5 of the pH titration curves. The complexation of TCPs with heavy metals was evaluated by stability constants (βn) of TCP-metal complexes whose stoichiometry was found to be 1:1 (ML) and 1:2 (ML2). TCPs from degree of hydrolysis of 25% hydrolysates gave high affinities towards Hg2+, Cd2+, and Pb2+ (giving similar or even bigger lgβ values than that of glutathione). A significantly positive correlation was found between the logarithm of stability constants for ML2 (lgβ2) and the sulfhydryl group content. Molecular weight distribution of TCPs affected the complexation with Pb2+ notably more than Hg2+ and Cd2+. These results suggest that soy TCPs have the potential to be used in the formulation of functional foods to counteract heavy metal accumulation in humans.
Figures



Similar articles
-
Selective Extraction and Antioxidant Properties of Thiol-Containing Peptides in Soy Glycinine Hydrolysates.Molecules. 2018 Jul 31;23(8):1909. doi: 10.3390/molecules23081909. Molecules. 2018. PMID: 30065200 Free PMC article.
-
Separation and evaluation of soybean protein hydrolysates prepared by immobilized metal ion affinity chromatography with different metal ions.J Chromatogr Sci. 2012 Sep;50(8):714-20. doi: 10.1093/chromsci/bms071. Epub 2012 May 25. J Chromatogr Sci. 2012. PMID: 22634192
-
Beta-conglycinin embeds active peptides that inhibit lipid accumulation in 3T3-L1 adipocytes in vitro.J Agric Food Chem. 2008 Nov 26;56(22):10533-43. doi: 10.1021/jf802216b. J Agric Food Chem. 2008. PMID: 18947234
-
Formation and characterization of amyloid-like fibrils from soy β-conglycinin and glycinin.J Agric Food Chem. 2010 Oct 27;58(20):11058-66. doi: 10.1021/jf1021658. Epub 2010 Oct 4. J Agric Food Chem. 2010. PMID: 20919718
-
Structural and functional analysis of various globulin proteins from soy seed.Crit Rev Food Sci Nutr. 2015;55(11):1491-502. doi: 10.1080/10408398.2012.700340. Crit Rev Food Sci Nutr. 2015. PMID: 24915310 Review.
Cited by
-
Turning Food Protein Waste into Sustainable Technologies.Chem Rev. 2023 Mar 8;123(5):2112-2154. doi: 10.1021/acs.chemrev.2c00236. Epub 2022 Jun 30. Chem Rev. 2023. PMID: 35772093 Free PMC article. Review.
-
Alleviating Effect of Methionine on Intestinal Development and Intercellular Junction Induced by Nickel.Biol Trace Elem Res. 2022 Sep;200(9):4007-4016. doi: 10.1007/s12011-021-02992-9. Epub 2021 Nov 5. Biol Trace Elem Res. 2022. PMID: 34739676
-
Benzylaminopurine and Abscisic Acid Mitigates Cadmium and Copper Toxicity by Boosting Plant Growth, Antioxidant Capacity, Reducing Metal Accumulation and Translocation in Bamboo [Pleioblastus pygmaeus (Miq.)] Plants.Antioxidants (Basel). 2022 Nov 24;11(12):2328. doi: 10.3390/antiox11122328. Antioxidants (Basel). 2022. PMID: 36552536 Free PMC article.
-
Lead detoxification of edible fungi Auricularia auricula and Pleurotus ostreatus: the purification of the chelation substances and their effects on rats.Front Nutr. 2023 Apr 20;10:1162110. doi: 10.3389/fnut.2023.1162110. eCollection 2023. Front Nutr. 2023. PMID: 37153916 Free PMC article.
-
Exogenous Glutathione Enhances Mercury Tolerance by Inhibiting Mercury Entry into Plant Cells.Front Plant Sci. 2017 May 1;8:683. doi: 10.3389/fpls.2017.00683. eCollection 2017. Front Plant Sci. 2017. PMID: 28507557 Free PMC article.
References
-
- Singh A., Ward O.P. Biodegradation and Bioremediation. Springer; Berlin, Germany: 2004. p. 237.
-
- Bae W., Chen W., Mulchandani A., Mehra R.K. Enhanced bioaccumulation of heavy metals by bacterial cells displaying synthetic phytochelatins. Biotechnol. Bioeng. 2000;70:518–524. - PubMed
-
- Meister A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988;263:17205–17208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

