Bioluminescence resonance energy transfer to detect protein-protein interactions in live cells
- PMID: 25859969
- PMCID: PMC4682348
- DOI: 10.1007/978-1-4939-2425-7_30
Bioluminescence resonance energy transfer to detect protein-protein interactions in live cells
Abstract
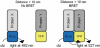
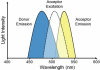
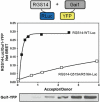
Bioluminescence resonance energy transfer (BRET) is a valuable tool to detect protein-protein interactions. BRET utilizes bioluminescent and fluorescent protein tags with compatible emission and excitation properties, making it possible to examine resonance energy transfer when the tags are in close proximity (<10 nm) as a typical result of protein-protein interactions. Here we describe a protocol for detecting BRET from two known protein binding partners (Gαi1 and RGS14) in HEK 293 cells using Renilla luciferase and yellow fluorescent protein tags. We discuss the calculation of the acceptor/donor ratio as well as net BRET and demonstrate that BRET can be used as a platform to investigate the regulation of protein-protein interactions in live cells in real time.
Figures
Similar articles
-
BRET: NanoLuc-Based Bioluminescence Resonance Energy Transfer Platform to Monitor Protein-Protein Interactions in Live Cells.Methods Mol Biol. 2016;1439:263-71. doi: 10.1007/978-1-4939-3673-1_17. Methods Mol Biol. 2016. PMID: 27317001
-
Use of BRET to Study Protein-Protein Interactions In Vitro and In Vivo.Methods Mol Biol. 2016;1443:57-78. doi: 10.1007/978-1-4939-3724-0_5. Methods Mol Biol. 2016. PMID: 27246334
-
Assembly and function of the regulator of G protein signaling 14 (RGS14)·H-Ras signaling complex in live cells are regulated by Gαi1 and Gαi-linked G protein-coupled receptors.J Biol Chem. 2013 Feb 1;288(5):3620-31. doi: 10.1074/jbc.M112.440057. Epub 2012 Dec 17. J Biol Chem. 2013. PMID: 23250758 Free PMC article.
-
Monitoring Ligand-Activated Protein-Protein Interactions Using Bioluminescent Resonance Energy Transfer (BRET) Assay.Methods Mol Biol. 2016;1473:3-15. doi: 10.1007/978-1-4939-6346-1_1. Methods Mol Biol. 2016. PMID: 27518618 Review.
-
The BRET technology and its application to screening assays.Biotechnol J. 2008 Mar;3(3):311-24. doi: 10.1002/biot.200700222. Biotechnol J. 2008. PMID: 18228541 Review.
Cited by
-
Current Approaches Toward Quantitative Mapping of the Interactome.Front Genet. 2016 May 4;7:74. doi: 10.3389/fgene.2016.00074. eCollection 2016. Front Genet. 2016. PMID: 27200083 Free PMC article.
-
RGS14 regulates the lifetime of Gα-GTP signaling but does not prolong Gβγ signaling following receptor activation in live cells.Pharmacol Res Perspect. 2016 Aug 18;4(5):e00249. doi: 10.1002/prp2.249. eCollection 2016 Oct. Pharmacol Res Perspect. 2016. PMID: 27713821 Free PMC article.
-
Molecular Evidence of Adenosine Deaminase Linking Adenosine A2A Receptor and CD26 Proteins.Front Pharmacol. 2018 Feb 15;9:106. doi: 10.3389/fphar.2018.00106. eCollection 2018. Front Pharmacol. 2018. PMID: 29497379 Free PMC article.
-
Genetic Analysis of Rare Human Variants of Regulators of G Protein Signaling Proteins and Their Role in Human Physiology and Disease.Pharmacol Rev. 2018 Jul;70(3):446-474. doi: 10.1124/pr.117.015354. Pharmacol Rev. 2018. PMID: 29871944 Free PMC article. Review.
-
Designing Chimeric Molecules for Drug Discovery by Leveraging Chemical Biology.J Med Chem. 2020 Mar 12;63(5):1908-1928. doi: 10.1021/acs.jmedchem.9b01456. Epub 2020 Feb 19. J Med Chem. 2020. PMID: 32023055 Free PMC article. Review.
References
-
- Wu P, Brand L. Resonance energy transfer: methods and applications. Anal Biochem. 1994;218:1–13. - PubMed
-
- Xu Y, Kanauchi A, von Arnim AG, et al. Bioluminescence resonance energy transfer: monitoring protein-protein interactions in living cells. Methods Enzymol. 2003;360:289–301. - PubMed
-
- Lohse MJ, Nuber S, Hoffmann C. Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein-coupled receptor activation and signaling. Pharmacol Rev. 2012;64:299–336. - PubMed
-
- Nagai T, Ibata K, Park ES, et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

