The Nurr1 Activator 1,1-Bis(3'-Indolyl)-1-(p-Chlorophenyl)Methane Blocks Inflammatory Gene Expression in BV-2 Microglial Cells by Inhibiting Nuclear Factor κB
- PMID: 25858541
- PMCID: PMC4429718
- DOI: 10.1124/mol.114.095398
The Nurr1 Activator 1,1-Bis(3'-Indolyl)-1-(p-Chlorophenyl)Methane Blocks Inflammatory Gene Expression in BV-2 Microglial Cells by Inhibiting Nuclear Factor κB
Abstract
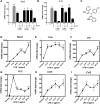
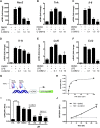
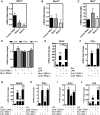
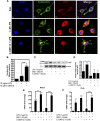
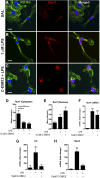
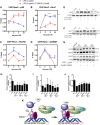
NR4A family orphan nuclear receptors are an important class of transcription factors for development and homeostasis of dopaminergic neurons that also inhibit expression of inflammatory genes in glial cells. The identification of NR4A2 (Nurr1) as a suppressor of nuclear factor κB (NF-κB)-related neuroinflammatory genes in microglia and astrocytes suggests that this receptor could be a target for pharmacologic intervention in neurologic disease, but compounds that promote this activity are lacking. Selected diindolylmethane compounds (C-DIMs) have been shown to activate or inactivate nuclear receptors, including Nurr1, in cancer cells and also suppress astrocyte inflammatory signaling in vitro. Based upon these data, we postulated that C-DIM12 [1,1-bis(3'-indolyl)-1-(p-chlorophenyl) methane] would suppress inflammatory signaling in microglia by a Nurr1-dependent mechanism. C-DIM12 inhibited lipopolysaccharide (LPS)-induced expression of NF-κB-regulated genes in BV-2 microglia including nitric oxide synthase (NOS2), interleukin-6 (IL-6), and chemokine (C-C motif) ligand 2 (CCL2), and the effects were attenuated by Nurr1-RNA interference. Additionally, C-DIM12 decreased NF-κB activation in NF-κB-GFP (green fluorescent protein) reporter cells and enhanced nuclear translocation of Nurr1 primary microglia. Chromatin immunoprecipitation assays indicated that C-DIM12 decreased lipopolysaccharide-induced p65 binding to the NOS2 promoter and concurrently enhanced binding of Nurr1 to the p65-binding site. Consistent with these findings, C-DIM12 also stabilized binding of the Corepressor for Repressor Element 1 Silencing Transcription Factor (CoREST) and the Nuclear Receptor Corepressor 2 (NCOR2). Collectively, these data identify C-DIM12 as a modulator of Nurr1 activity that results in inhibition of NF-κB-dependent gene expression in glial cells by stabilizing nuclear corepressor proteins, which reduces binding of p65 to inflammatory gene promoters.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
Similar articles
-
A novel diindolylmethane analog, 1,1-bis(3'-indolyl)-1-(p-chlorophenyl) methane, inhibits the tumor necrosis factor-induced inflammatory response in primary murine synovial fibroblasts through a Nurr1-dependent mechanism.Mol Immunol. 2018 Sep;101:46-54. doi: 10.1016/j.molimm.2018.05.024. Epub 2018 Jun 2. Mol Immunol. 2018. PMID: 29870816 Free PMC article.
-
Structurally Distinct Nurr1 Ligands Exhibit Different Pharmacological Characteristics in Regulating Inflammatory Responses of Microglial BV-2 Cells.Biol Pharm Bull. 2024;47(11):1937-1945. doi: 10.1248/bpb.b24-00644. Biol Pharm Bull. 2024. PMID: 39603614
-
The Nurr1 Ligand,1,1-bis(3'-Indolyl)-1-(p-Chlorophenyl)Methane, Modulates Glial Reactivity and Is Neuroprotective in MPTP-Induced Parkinsonism.J Pharmacol Exp Ther. 2018 Jun;365(3):636-651. doi: 10.1124/jpet.117.246389. Epub 2018 Apr 6. J Pharmacol Exp Ther. 2018. PMID: 29626009 Free PMC article.
-
Structure-dependent activation of gene expression by bis-indole and quinoline-derived activators of nuclear receptor 4A2.Chem Biol Drug Des. 2019 Oct;94(4):1711-1720. doi: 10.1111/cbdd.13564. Epub 2019 Jul 21. Chem Biol Drug Des. 2019. PMID: 31102570 Free PMC article. Review.
-
NURR1 Impairment in Multiple Sclerosis.Int J Mol Sci. 2019 Sep 30;20(19):4858. doi: 10.3390/ijms20194858. Int J Mol Sci. 2019. PMID: 31574937 Free PMC article. Review.
Cited by
-
Nuclear receptor 4A2 (NR4A2) is a druggable target for glioblastomas.J Neurooncol. 2020 Jan;146(1):25-39. doi: 10.1007/s11060-019-03349-y. Epub 2019 Nov 21. J Neurooncol. 2020. PMID: 31754919 Free PMC article.
-
Striatal Nurr1 Facilitates the Dyskinetic State and Exacerbates Levodopa-Induced Dyskinesia in a Rat Model of Parkinson's Disease.J Neurosci. 2020 Apr 29;40(18):3675-3691. doi: 10.1523/JNEUROSCI.2936-19.2020. Epub 2020 Apr 1. J Neurosci. 2020. PMID: 32238479 Free PMC article.
-
The Nurr1 ligand indole acetic acid hydrazide loaded onto ZnFe2O4 nanoparticles suppresses proinflammatory gene expressions in SimA9 microglial cells.Sci Rep. 2024 Jun 17;14(1):13987. doi: 10.1038/s41598-024-64820-z. Sci Rep. 2024. PMID: 38886466 Free PMC article.
-
LDOC1 as Negative Prognostic Marker for Vulvar Cancer Patients.Int J Mol Sci. 2020 Dec 5;21(23):9287. doi: 10.3390/ijms21239287. Int J Mol Sci. 2020. PMID: 33291445 Free PMC article.
-
A novel diindolylmethane analog, 1,1-bis(3'-indolyl)-1-(p-chlorophenyl) methane, inhibits the tumor necrosis factor-induced inflammatory response in primary murine synovial fibroblasts through a Nurr1-dependent mechanism.Mol Immunol. 2018 Sep;101:46-54. doi: 10.1016/j.molimm.2018.05.024. Epub 2018 Jun 2. Mol Immunol. 2018. PMID: 29870816 Free PMC article.
References
-
- Bensinger SJ, Tontonoz P. (2009) A Nurr1 pathway for neuroprotection. Cell 137:26–28. - PubMed
-
- Buss H, Dörrie A, Schmitz ML, Frank R, Livingstone M, Resch K, Kracht M. (2004) Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem 279:49571–49574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

