miR-26a and miR-384-5p are required for LTP maintenance and spine enlargement
- PMID: 25858512
- PMCID: PMC4403380
- DOI: 10.1038/ncomms7789
miR-26a and miR-384-5p are required for LTP maintenance and spine enlargement
Abstract
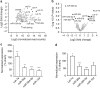
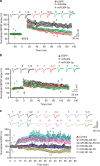
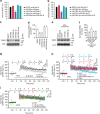
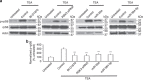
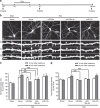
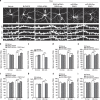
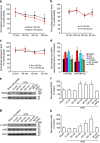
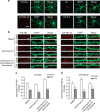
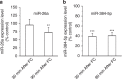
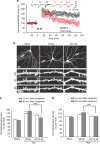
Long-term potentiation (LTP) is a form of synaptic plasticity that results in enhanced synaptic strength. It is associated with the formation and enlargement of dendritic spines-tiny protrusions accommodating excitatory synapses. Both LTP and spine remodelling are crucial for brain development, cognition and the pathophysiology of neurological disorders. The role of microRNAs (miRNAs) in the maintenance of LTP, however, is not well understood. Using next-generation sequencing to profile miRNA transcriptomes, we demonstrate that miR-26a and miR-384-5p specifically affect the maintenance, but not induction, of LTP and different stages of spine enlargement by regulating the expression of RSK3. Using bioinformatics, we also examine the global effects of miRNA transcriptome changes during LTP on gene expression and cellular activities. This study reveals a novel miRNA-mediated mechanism for gene-specific regulation of translation in LTP, identifies two miRNAs required for long-lasting synaptic and spine plasticity and presents a catalogue of candidate 'LTP miRNAs'.
Figures
Similar articles
-
Shifting patterns of polyribosome accumulation at synapses over the course of hippocampal long-term potentiation.Hippocampus. 2018 Jun;28(6):416-430. doi: 10.1002/hipo.22841. Epub 2018 Apr 16. Hippocampus. 2018. PMID: 29575288 Free PMC article.
-
Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP.Hippocampus. 2011 Apr;21(4):354-73. doi: 10.1002/hipo.20768. Hippocampus. 2011. PMID: 20101601 Free PMC article.
-
Deletion of LRRTM1 and LRRTM2 in adult mice impairs basal AMPA receptor transmission and LTP in hippocampal CA1 pyramidal neurons.Proc Natl Acad Sci U S A. 2018 Jun 5;115(23):E5382-E5389. doi: 10.1073/pnas.1803280115. Epub 2018 May 21. Proc Natl Acad Sci U S A. 2018. PMID: 29784826 Free PMC article.
-
A role for the spine apparatus in LTP and spatial learning.Behav Brain Res. 2008 Sep 1;192(1):12-9. doi: 10.1016/j.bbr.2008.02.033. Epub 2008 Feb 26. Behav Brain Res. 2008. PMID: 18395274 Review.
-
Voltage Imaging in the Study of Hippocampal Circuit Function and Plasticity.Adv Exp Med Biol. 2015;859:197-211. doi: 10.1007/978-3-319-17641-3_8. Adv Exp Med Biol. 2015. PMID: 26238054 Review.
Cited by
-
Temporospatial guidance of activity-dependent gene expression by microRNA: mechanisms and functional implications for neural plasticity.Nucleic Acids Res. 2019 Jan 25;47(2):533-545. doi: 10.1093/nar/gky1235. Nucleic Acids Res. 2019. PMID: 30535081 Free PMC article. Review.
-
Human Adult Astrocyte Extracellular Vesicle Transcriptomics Study Identifies Specific RNAs Which Are Preferentially Secreted as EV Luminal Cargo.Genes (Basel). 2023 Mar 31;14(4):853. doi: 10.3390/genes14040853. Genes (Basel). 2023. PMID: 37107614 Free PMC article.
-
Role of MicroRNA in Governing Synaptic Plasticity.Neural Plast. 2016;2016:4959523. doi: 10.1155/2016/4959523. Epub 2016 Mar 13. Neural Plast. 2016. PMID: 27034846 Free PMC article. Review.
-
Temporal dynamics of miRNAs in human DLPFC and its association with miRNA dysregulation in schizophrenia.Transl Psychiatry. 2019 Aug 20;9(1):196. doi: 10.1038/s41398-019-0538-y. Transl Psychiatry. 2019. PMID: 31431609 Free PMC article.
-
Control of macrophage autophagy by miR-384-5p in the development of diabetic encephalopathy.Am J Transl Res. 2018 Feb 15;10(2):511-518. eCollection 2018. Am J Transl Res. 2018. PMID: 29511445 Free PMC article.
References
-
- Whitlock J. R., Heynen A. J., Shuler M. G. & Bear M. F. Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097 (2006) . - PubMed
-
- Pastalkova E. et al. Storage of spatial information by the maintenance mechanism of LTP. Science 313, 1141–1144 (2006) . - PubMed
-
- Kelleher R. J. 3rd, Govindarajan A. & Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron 44, 59–73 (2004) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

