Mitophagy and the mitochondrial unfolded protein response in neurodegeneration and bacterial infection
- PMID: 25857750
- PMCID: PMC4384303
- DOI: 10.1186/s12915-015-0129-1
Mitophagy and the mitochondrial unfolded protein response in neurodegeneration and bacterial infection
Abstract
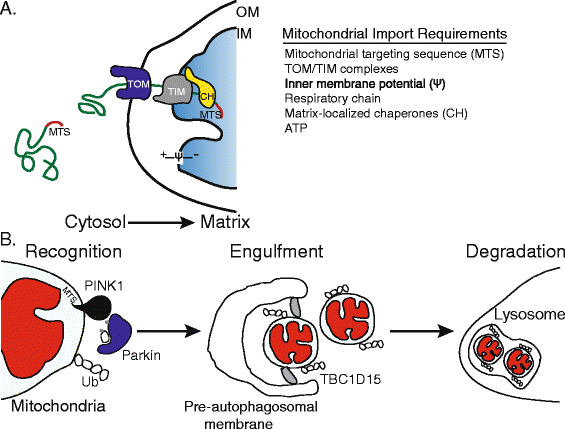
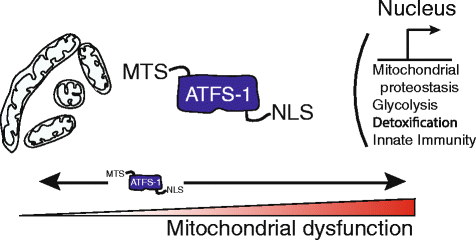
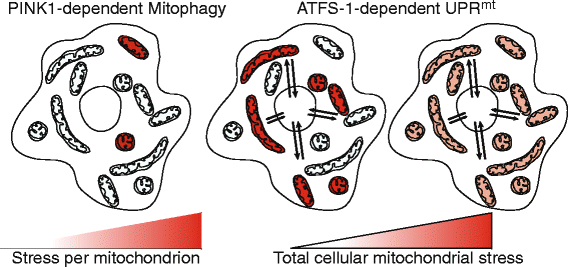
Mitochondria are highly dynamic and structurally complex organelles that provide multiple essential metabolic functions. Mitochondrial dysfunction is associated with neurodegenerative conditions such as Parkinson's disease, as well as bacterial infection. Here, we explore the roles of mitochondrial autophagy (mitophagy) and the mitochondrial unfolded protein response (UPR(mt)) in the response to mitochondrial dysfunction, focusing in particular on recent evidence on the role of mitochondrial import efficiency in the regulation of these stress pathways and how they may interact to protect the mitochondrial pool while initiating an innate immune response to protect against bacterial pathogens.
Figures
Similar articles
-
Dysregulation of the Mitochondrial Unfolded Protein Response Induces Non-Apoptotic Dopaminergic Neurodegeneration in C. elegans Models of Parkinson's Disease.J Neurosci. 2017 Nov 15;37(46):11085-11100. doi: 10.1523/JNEUROSCI.1294-17.2017. Epub 2017 Oct 13. J Neurosci. 2017. PMID: 29030433 Free PMC article.
-
Mitophagy and neurodegeneration: the zebrafish model system.Autophagy. 2013 Nov 1;9(11):1693-709. doi: 10.4161/auto.25082. Epub 2013 Aug 8. Autophagy. 2013. PMID: 23939015 Review.
-
On the offense and defense: mitochondrial recovery programs amidst targeted pathogenic assault.FEBS J. 2022 Nov;289(22):7014-7037. doi: 10.1111/febs.16126. Epub 2021 Jul 30. FEBS J. 2022. PMID: 34270874 Free PMC article. Review.
-
The mitochondrial unfolded protein response and its diverse roles in cellular stress.Int J Biochem Cell Biol. 2021 Apr;133:105934. doi: 10.1016/j.biocel.2021.105934. Epub 2021 Jan 30. Int J Biochem Cell Biol. 2021. PMID: 33529716 Review.
-
The intersection of exercise and aging on mitochondrial protein quality control.Exp Gerontol. 2020 Mar;131:110824. doi: 10.1016/j.exger.2019.110824. Epub 2020 Jan 3. Exp Gerontol. 2020. PMID: 31911185 Review.
Cited by
-
Molecular Hydrogen Enhances Proliferation of Cancer Cells That Exhibit Potent Mitochondrial Unfolded Protein Response.Int J Mol Sci. 2022 Mar 7;23(5):2888. doi: 10.3390/ijms23052888. Int J Mol Sci. 2022. PMID: 35270030 Free PMC article.
-
Autophagic flux induced by graphene oxide has a neuroprotective effect against human prion protein fragments.Int J Nanomedicine. 2017 Nov 8;12:8143-8158. doi: 10.2147/IJN.S146398. eCollection 2017. Int J Nanomedicine. 2017. PMID: 29184404 Free PMC article.
-
A quantitative genome-wide RNAi screen in C. elegans for antifungal innate immunity genes.BMC Biol. 2016 Apr 29;14:35. doi: 10.1186/s12915-016-0256-3. BMC Biol. 2016. PMID: 27129311 Free PMC article.
-
Mitochondrial proteostasis in the context of cellular and organismal health and aging.J Biol Chem. 2019 Apr 5;294(14):5396-5407. doi: 10.1074/jbc.TM117.000893. Epub 2018 Apr 5. J Biol Chem. 2019. PMID: 29622680 Free PMC article. Review.
-
Interfacing mitochondrial biogenesis and elimination to enhance host pathogen defense and longevity.Worm. 2015 Jul 29;4(3):e1071763. doi: 10.1080/21624054.2015.1071763. eCollection 2015 Jul-Sep. Worm. 2015. PMID: 26430570 Free PMC article.
References
-
- van der Laan M, Hutu DP, Rehling P. On the mechanism of preprotein import by the mitochondrial presequence translocase. Biochim Biophys Acta. 1803;2010:732–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

