Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality
- PMID: 25856755
- PMCID: PMC4392343
- DOI: 10.1016/j.chom.2015.03.002
Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality
Abstract
In addition to direct effects on virus infectivity, antibodies mediate antibody-dependent cellular cytotoxicity (ADCC), the killing of an antibody-coated virus-infected cell by cytotoxic effector cells. Although ADCC has been suggested to protect against HIV, the relationship between HIV-specific ADCC antibodies at the time of HIV exposure and infection outcome in humans remains to be assessed. We evaluated the ADCC activity of passively acquired antibodies in infants born to HIV-infected mothers. ADCC levels were higher in uninfected than infected infants, although not significantly. Increase in ADCC antibody activity in infected infants was associated with reduced mortality risk. Infant ADCC positively correlated with the magnitude of IgG1 binding, and IgG1 levels were associated with survival in infected infants. Infant IgG3-binding antibodies were not associated with infected infant survival. These data suggest a therapeutic benefit of pre-existing HIV-specific ADCC antibodies and support a role for eliciting ADCC-mediating IgG1 in HIV vaccines.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures

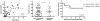
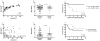
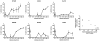
Similar articles
-
Antibody-dependent cellular cytotoxicity targeting CD4-inducible epitopes predicts mortality in HIV-infected infants.EBioMedicine. 2019 Sep;47:257-268. doi: 10.1016/j.ebiom.2019.08.072. EBioMedicine. 2019. PMID: 31501077 Free PMC article.
-
Improved HIV-positive infant survival is correlated with high levels of HIV-specific ADCC activity in multiple cohorts.Cell Rep Med. 2021 Apr 20;2(4):100254. doi: 10.1016/j.xcrm.2021.100254. eCollection 2021 Apr 20. Cell Rep Med. 2021. PMID: 33948582 Free PMC article.
-
Delayed generation of antibodies mediating human immunodeficiency virus type 1-specific antibody-dependent cellular cytotoxicity in vertically infected infants. WITS Study Group. Women and Infants Transmission Study.J Infect Dis. 1997 Sep;176(3):643-8. doi: 10.1086/514085. J Infect Dis. 1997. PMID: 9291310
-
Anti-HIV-1 antibody-dependent cellular cytotoxicity: is there more to antibodies than neutralization?Curr Opin HIV AIDS. 2018 Mar;13(2):160-166. doi: 10.1097/COH.0000000000000439. Curr Opin HIV AIDS. 2018. PMID: 29194123 Review.
-
Antibody-dependent cellular cytotoxicity in HIV infections.FASEB J. 1996 Feb;10(2):258-66. doi: 10.1096/fasebj.10.2.8641559. FASEB J. 1996. PMID: 8641559 Review.
Cited by
-
Identification of a HIV Gp41-Specific Human Monoclonal Antibody With Potent Antibody-Dependent Cellular Cytotoxicity.Front Immunol. 2018 Nov 16;9:2613. doi: 10.3389/fimmu.2018.02613. eCollection 2018. Front Immunol. 2018. PMID: 30519238 Free PMC article.
-
Identification of HIV gp41-specific antibodies that mediate killing of infected cells.PLoS Pathog. 2019 Feb 19;15(2):e1007572. doi: 10.1371/journal.ppat.1007572. eCollection 2019 Feb. PLoS Pathog. 2019. PMID: 30779811 Free PMC article.
-
Diversity and Function of Maternal HIV-1-Specific Antibodies at the Time of Vertical Transmission.J Virol. 2020 Apr 16;94(9):e01594-19. doi: 10.1128/JVI.01594-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32075936 Free PMC article.
-
Engineering broadly neutralizing antibodies for HIV prevention and therapy.Adv Drug Deliv Rev. 2016 Aug 1;103:157-173. doi: 10.1016/j.addr.2016.01.013. Epub 2016 Jan 29. Adv Drug Deliv Rev. 2016. PMID: 26827912 Free PMC article. Review.
-
Patterns of conserved gp120 epitope presentation on attached HIV-1 virions.Proc Natl Acad Sci U S A. 2017 Nov 14;114(46):E9893-E9902. doi: 10.1073/pnas.1705074114. Epub 2017 Oct 30. Proc Natl Acad Sci U S A. 2017. PMID: 29087304 Free PMC article.
References
-
- Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, et al. Polyfunctional Fc-Effector Profiles Mediated by IgG Subclass Selection Distinguish RV144 and VAX003 Vaccines. Sci Transl Med. 2014;6:228ra38. - PubMed
-
- Ferrante A, Beard LJ, Feldman RG. IgG subclass distribution of antibodies to bacterial and viral antigens. Ped Infect Dis J. 1990;9:S16–S24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

