Reductions in Use of Colchicine after FDA Enforcement of Market Exclusivity in a Commercially Insured Population
- PMID: 25855479
- PMCID: PMC4617917
- DOI: 10.1007/s11606-015-3285-7
Reductions in Use of Colchicine after FDA Enforcement of Market Exclusivity in a Commercially Insured Population
Abstract
Background: A brand-name version of colchicine (Colcrys) was introduced after its manufacturer conducted a clinical trial in acute gout patients, leading to higher prices for this drug.
Objective: We analyzed the impact of the new single-source colchicine product on prescribing and patient health spending as well as incidence rates of potentially dangerous concomitant use of clarithromycin and cyclosporine after formal FDA approval.
Design/participants: We conducted a retrospective cohort study of UnitedHealth-affiliated enrollees newly diagnosed with gout or FMF.
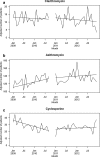
Main measures: Among gout and FMF patients separately, we assessed linear trends in colchicine prescriptions, prescription drug costs, and total health care costs from 2009 to September 2010 (market exclusivity announced) compared to January 2011 (market exclusivity enforced) through 2012. Next, we estimated trends in co-prescription within 15 days of clarithromycin, azithromycin (indicated on the Colcrys label for use in place of clarithromycin), and cyclosporine.
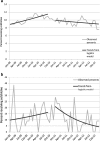
Key results: Among gout patients, before Colcrys' market exclusivity, the odds of receiving colchicine within 30 days of gout diagnosis increased 1.4 %/month (OR: 1.014, 95 % CI: 1.011-1.018). Following FDA action, the odds decreased by 0.5 %/month (OR: 0.995, 95 % CI: 0.992-0.999) (p < 0.001). Similarly, among FMF patients, odds of initiating colchicine changed from an increase of 2.8 %/month to a decrease by 7.6 %/month (p = 0.01). Patients receiving colchicine experienced increases in average monthly prescription drug costs ($418 vs. $651, p < 0.001) and health care costs ($3,406 vs. $3,534, p < 0.001). Incidence rates of colchicine/clarithromycin co-prescription before and after FDA action did not change, while co-prescription of colchicine/cyclosporine increased after introduction of Colcrys [-0.75 monthly change in patients (95 % CI: -1.07, -0.43) vs. 0.13 (95 % CI: -0.16, 0.42), p < 0.001].
Conclusions: The FDA's actions were associated with a reduction in colchicine initiation and an increase in patient spending. By contrast, we did not observe any association with improvements in avoidance of potentially dangerous co-prescriptions.
Figures
Comment in
-
Good Intentions, Unintended Consequences, and Unrealized Benefits.J Gen Intern Med. 2015 Nov;30(11):1581-3. doi: 10.1007/s11606-015-3458-4. J Gen Intern Med. 2015. PMID: 26239629 Free PMC article. No abstract available.
Similar articles
-
Effect of Generic Competition on Atorvastatin Prescribing and Patients' Out-of-Pocket Spending.JAMA Intern Med. 2016 Sep 1;176(9):1317-23. doi: 10.1001/jamainternmed.2016.3384. JAMA Intern Med. 2016. PMID: 27367749
-
Colchicine for the treatment of gout.Expert Opin Pharmacother. 2010 Dec;11(17):2933-8. doi: 10.1517/14656566.2010.529432. Expert Opin Pharmacother. 2010. PMID: 21050036 Review.
-
A new perspective on the pharmacoeconomics of colchicine.Curr Med Res Opin. 2011 May;27(5):931-7. doi: 10.1185/03007995.2011.563284. Epub 2011 Mar 3. Curr Med Res Opin. 2011. PMID: 21370937 Review.
-
Benefit restrictions and gout treatment.J Manag Care Pharm. 2013 Nov-Dec;19(9):773-82. doi: 10.18553/jmcp.2013.19.9.773. J Manag Care Pharm. 2013. PMID: 24156646 Free PMC article.
-
Nonsteroidal anti-inflammatory drugs and colchicine to prevent gout flare during early urate-lowering therapy: perspectives on alternative therapies and costs.J Pain Palliat Care Pharmacother. 2010 Dec;24(4):402-4. doi: 10.3109/15360288.2010.526174. J Pain Palliat Care Pharmacother. 2010. PMID: 21133751
Cited by
-
Colchicine intoxication in familial Mediterranean fever patients using clarithromycin for the treatment of Helicobacter pylori: a series of six patients.Rheumatol Int. 2018 Jan;38(1):141-147. doi: 10.1007/s00296-017-3823-1. Epub 2017 Oct 3. Rheumatol Int. 2018. PMID: 28975396
-
Inflammation May be the Future of Cardiovascular Risk Reduction: Does Colchicine have a Current Indication?Am J Cardiovasc Drugs. 2021 Jan;21(1):1-10. doi: 10.1007/s40256-020-00408-y. Am J Cardiovasc Drugs. 2021. PMID: 32356107 Review.
-
Something old, something new: a paradigm for considering immune therapies for cardiovascular disease.Cardiovasc Res. 2020 Apr 1;116(5):e51-e53. doi: 10.1093/cvr/cvaa055. Cardiovasc Res. 2020. PMID: 32215658 Free PMC article. No abstract available.
-
Cilostazol for Secondary Stroke Prevention: History, Evidence, Limitations, and Possibilities.Stroke. 2021 Oct;52(10):e635-e645. doi: 10.1161/STROKEAHA.121.035002. Epub 2021 Sep 14. Stroke. 2021. PMID: 34517768 Free PMC article. Review.
-
The FDA Unapproved Drugs Initiative: An Observational Study of the Consequences for Drug Prices and Shortages in the United States.J Manag Care Spec Pharm. 2017 Oct;23(10):1066-1076. doi: 10.18553/jmcp.2017.23.10.1066. J Manag Care Spec Pharm. 2017. PMID: 28944731 Free PMC article.
References
-
- Carpenter D. Reputation and Power: Organizational Image and Pharmaceutical Regulation at the FDA. Princeton: Princeton Univ Press; 2010.
-
- Food and Drug Administration. Unapproved drugs initiative. Jan 5 2011. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Enforce....
-
- Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: twenty-four–hour outcome of the first multicenter, randomized, double-blind, placebo- controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060–8. doi: 10.1002/art.27327. - DOI - PubMed
-
- Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Liote F, McCarthy G, Netter P, Nuki G, Perez-Ruiz F, Pignone A, Pimentao J, Punzi L, Roddy E, Uhlig T, Zimmermann-Gorska I. EULAR evidence based recommendations for gout, part II: management. Ann Rheum Dis. 2006;65(10):1312–24. doi: 10.1136/ard.2006.055269. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

