PLK1 and HOTAIR Accelerate Proteasomal Degradation of SUZ12 and ZNF198 during Hepatitis B Virus-Induced Liver Carcinogenesis
- PMID: 25855382
- PMCID: PMC4452430
- DOI: 10.1158/0008-5472.CAN-14-2928
PLK1 and HOTAIR Accelerate Proteasomal Degradation of SUZ12 and ZNF198 during Hepatitis B Virus-Induced Liver Carcinogenesis
Abstract
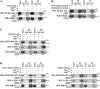
Elucidating mechanisms of hepatitis B virus (HBV)-mediated hepatocarcinogenesis is needed to gain insights into the etiology and treatment of liver cancer. Cells where HBV is replicating exhibit increased expression of Plk1 kinase and reduced levels of two transcription repression factors, SUZ12 and ZNF198. SUZ12 is an essential subunit of the transcription repressive complex PRC2. ZNF198 stabilizes the transcription repressive complex composed of LSD1, Co-REST, and HDAC1. These two transcription repressive complexes are held together by binding the long noncoding RNA HOTAIR. In this study, we linked these regulatory events mechanistically by showing that Plk1 induces proteasomal degradation of SUZ12 and ZNF198 by site-specific phosphorylation. Plk1-dependent ubiquitination of SUZ12 and ZNF198 was enhanced by expression of HOTAIR, significantly reducing SUZ12 and ZNF198 stability. In cells expressing the HBV X protein (HBx), downregulation of SUZ12 and ZNF198 mediated global changes in histone modifications. In turn, HBx-expressing cells propagated an altered chromatin landscape after cell division, as exemplified by changes in histone modifications of the EpCAM promoter, a target of PRC2 and LSD1/Co-REST/HDAC1 complexes. Notably, liver tumors from X/c-myc bitransgenic mice exhibited downregulation of SUZ12 and ZNF198 along with elevated expression of Plk1, HOTAIR, and EpCAM. Clinically, similar effects were documented in a set of HBV-related liver tumors consistent with the likelihood that downregulation of SUZ12 and ZNF198 leads to epigenetic reprogramming of infected hepatocytes. Because both Plk1 and HOTAIR are elevated in many human cancers, we propose that their combined effects are involved in epigenetic reprogramming associated broadly with oncogenic transformation.
©2015 American Association for Cancer Research.
Figures
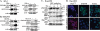




Similar articles
-
Subset of Suz12/PRC2 target genes is activated during hepatitis B virus replication and liver carcinogenesis associated with HBV X protein.Hepatology. 2012 Oct;56(4):1240-51. doi: 10.1002/hep.25781. Hepatology. 2012. PMID: 22505317 Free PMC article.
-
Proteins ZNF198 and SUZ12 are down-regulated in hepatitis B virus (HBV) X protein-mediated hepatocyte transformation and in HBV replication.Hepatology. 2011 Apr;53(4):1137-47. doi: 10.1002/hep.24163. Hepatology. 2011. PMID: 21480320 Free PMC article.
-
RNA helicase DEAD box protein 5 regulates Polycomb repressive complex 2/Hox transcript antisense intergenic RNA function in hepatitis B virus infection and hepatocarcinogenesis.Hepatology. 2016 Oct;64(4):1033-48. doi: 10.1002/hep.28698. Epub 2016 Aug 8. Hepatology. 2016. PMID: 27338022 Free PMC article.
-
Deregulation of epigenetic mechanisms by the hepatitis B virus X protein in hepatocarcinogenesis.Viruses. 2013 Mar 18;5(3):858-72. doi: 10.3390/v5030858. Viruses. 2013. PMID: 23507839 Free PMC article. Review.
-
Mechanisms of HBV-induced hepatocellular carcinoma.J Hepatol. 2016 Apr;64(1 Suppl):S84-S101. doi: 10.1016/j.jhep.2016.02.021. J Hepatol. 2016. PMID: 27084040 Review.
Cited by
-
Restoration of RNA helicase DDX5 suppresses hepatitis B virus (HBV) biosynthesis and Wnt signaling in HBV-related hepatocellular carcinoma.Theranostics. 2020 Sep 1;10(24):10957-10972. doi: 10.7150/thno.49629. eCollection 2020. Theranostics. 2020. PMID: 33042264 Free PMC article.
-
Long non-coding RNA IRAIN suppresses apoptosis and promotes proliferation by binding to LSD1 and EZH2 in pancreatic cancer.Tumour Biol. 2016 Nov;37(11):14929-14937. doi: 10.1007/s13277-016-5380-8. Epub 2016 Sep 19. Tumour Biol. 2016. PMID: 27644252
-
CK2-mediated phosphorylation of SUZ12 promotes PRC2 function by stabilizing enzyme active site.Nat Commun. 2022 Nov 9;13(1):6781. doi: 10.1038/s41467-022-34431-1. Nat Commun. 2022. PMID: 36351927 Free PMC article.
-
RNA helicase DDX5 enables STAT1 mRNA translation and interferon signalling in hepatitis B virus replicating hepatocytes.Gut. 2022 May;71(5):991-1005. doi: 10.1136/gutjnl-2020-323126. Epub 2021 May 21. Gut. 2022. PMID: 34021034 Free PMC article.
-
A New Understanding of Long Non-Coding RNA in Hepatocellular Carcinoma-From m6A Modification to Blood Biomarkers.Cells. 2023 Sep 14;12(18):2272. doi: 10.3390/cells12182272. Cells. 2023. PMID: 37759495 Free PMC article. Review.
References
-
- Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–33. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008;359:378–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

