Tonic firing rate controls dendritic Ca2+ signaling and synaptic gain in substantia nigra dopamine neurons
- PMID: 25855191
- PMCID: PMC4388935
- DOI: 10.1523/JNEUROSCI.3904-14.2015
Tonic firing rate controls dendritic Ca2+ signaling and synaptic gain in substantia nigra dopamine neurons
Abstract
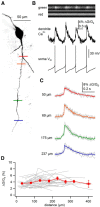
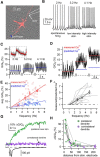
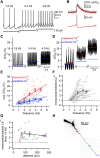
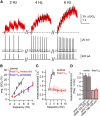
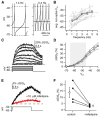
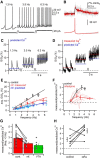
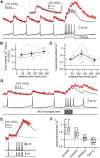
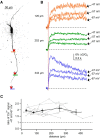
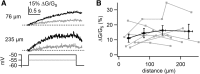
Substantia nigra dopamine neurons fire tonically resulting in action potential backpropagation and dendritic Ca(2+) influx. Using Ca(2+) imaging in acute mouse brain slices, we find a surprisingly steep relationship between tonic firing rate and dendritic Ca(2+). Increasing the tonic rate from 1 to 6 Hz generated Ca(2+) signals up to fivefold greater than predicted by linear summation of single spike-evoked Ca(2+)-transients. This "Ca(2+) supralinearity" was produced largely by depolarization of the interspike voltage leading to activation of subthreshold Ca(2+) channels and was present throughout the proximal and distal dendrites. Two-photon glutamate uncaging experiments show somatic depolarization enhances NMDA receptor-mediated Ca(2+) signals >400 μm distal to the soma, due to unusually tight electrotonic coupling of the soma to distal dendrites. Consequently, we find that fast tonic firing intensifies synaptically driven burst firing output in dopamine neurons. These results show that modulation of background firing rate precisely tunes dendritic Ca(2+) signaling and provides a simple yet powerful mechanism to dynamically regulate the gain of synaptic input.
Keywords: action potential; backpropagation; calcium imaging; dendrite; dopamine; substantia nigra.
Copyright © 2015 the authors 0270-6474/15/355823-14$15.00/0.
Figures

Similar articles
-
Homeostatic regulation mechanism of spontaneous firing determines glutamate responsiveness in the midbrain dopamine neurons.Cell Calcium. 2013 Oct;54(4):295-306. doi: 10.1016/j.ceca.2013.07.004. Epub 2013 Aug 12. Cell Calcium. 2013. PMID: 23988034
-
Functional organization of dendritic Ca2+ signals in midbrain dopamine neurons.Cell Calcium. 2011 Oct;50(4):370-80. doi: 10.1016/j.ceca.2011.06.007. Epub 2011 Jul 16. Cell Calcium. 2011. PMID: 21757230
-
Calcium dynamics control K-ATP channel-mediated bursting in substantia nigra dopamine neurons: a combined experimental and modeling study.J Neurophysiol. 2018 Jan 1;119(1):84-95. doi: 10.1152/jn.00351.2017. Epub 2017 Oct 4. J Neurophysiol. 2018. PMID: 28978764 Free PMC article.
-
Synaptic activation of dendritic AMPA and NMDA receptors generates transient high-frequency firing in substantia nigra dopamine neurons in vitro.J Neurophysiol. 2007 Apr;97(4):2837-50. doi: 10.1152/jn.01157.2006. Epub 2007 Jan 24. J Neurophysiol. 2007. PMID: 17251363
-
Current application and technology of functional multineuron calcium imaging.Biol Pharm Bull. 2009 Jan;32(1):1-9. doi: 10.1248/bpb.32.1. Biol Pharm Bull. 2009. PMID: 19122272 Review.
Cited by
-
Calcium, Bioenergetics, and Parkinson's Disease.Cells. 2020 Sep 8;9(9):2045. doi: 10.3390/cells9092045. Cells. 2020. PMID: 32911641 Free PMC article. Review.
-
Calcium and Parkinson's disease.Biochem Biophys Res Commun. 2017 Feb 19;483(4):1013-1019. doi: 10.1016/j.bbrc.2016.08.168. Epub 2016 Aug 30. Biochem Biophys Res Commun. 2017. PMID: 27590583 Free PMC article. Review.
-
Quantal Release of Dopamine and Action Potential Firing Detected in Midbrain Neurons by Multifunctional Diamond-Based Microarrays.Front Neurosci. 2019 Apr 9;13:288. doi: 10.3389/fnins.2019.00288. eCollection 2019. Front Neurosci. 2019. PMID: 31024230 Free PMC article.
-
Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):156-178. doi: 10.1111/jnc.13572. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26865375 Free PMC article. Review.
-
Role of the Axon Initial Segment in the Control of Spontaneous Frequency of Nigral Dopaminergic Neurons In Vivo.J Neurosci. 2018 Jan 17;38(3):733-744. doi: 10.1523/JNEUROSCI.1432-17.2017. Epub 2017 Dec 7. J Neurosci. 2018. PMID: 29217687 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
