Muscarinic receptors modulate dendrodendritic inhibitory synapses to sculpt glomerular output
- PMID: 25855181
- PMCID: PMC4388926
- DOI: 10.1523/JNEUROSCI.4953-14.2015
Muscarinic receptors modulate dendrodendritic inhibitory synapses to sculpt glomerular output
Abstract
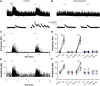
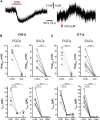
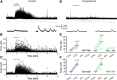
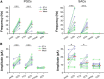
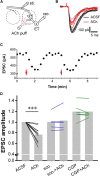
Cholinergic [acetylcholine (ACh)] axons from the basal forebrain innervate olfactory bulb glomeruli, the initial site of synaptic integration in the olfactory system. Both nicotinic acetylcholine receptors (nAChRs) and muscarinic acetylcholine receptors (mAChRs) are expressed in glomeruli. The activation of nAChRs directly excites both mitral/tufted cells (MTCs) and external tufted cells (ETCs), the two major excitatory neurons that transmit glomerular output. The functional roles of mAChRs in glomerular circuits are unknown. We show that the restricted glomerular application of ACh causes rapid, brief nAChR-mediated excitation of both MTCs and ETCs in the mouse olfactory bulb. This excitation is followed by mAChR-mediated inhibition, which is blocked by GABAA receptor antagonists, indicating the engagement of periglomerular cells (PGCs) and/or short axon cells (SACs), the two major glomerular inhibitory neurons. Indeed, selective activation of glomerular mAChRs, with ionotropic GluRs and nAChRs blocked, increased IPSCs in MTCs and ETCs, indicating that mAChRs recruit glomerular inhibitory circuits. Selective activation of glomerular mAChRs in the presence of tetrodotoxin increased IPSCs in all glomerular neurons, indicating action potential-independent enhancement of GABA release from PGC and/or SAC dendrodendritic synapses. mAChR-mediated enhancement of GABA release also presynaptically suppressed the first synapse of the olfactory system via GABAB receptors on sensory terminals. Together, these results indicate that cholinergic modulation of glomerular circuits is biphasic, involving an initial excitation of MTC/ETCs mediated by nAChRs followed by inhibition mediated directly by mAChRs on PGCs/SACs. This may phasically enhance the sensitivity of glomerular outputs to odorants, an action that is consistent with recent in vivo findings.
Keywords: cholinergic modulation; dendrodendritic synapses; glomerular circuits; glomerular output; inhibitory interneurons; olfactory bulb.
Copyright © 2015 the authors 0270-6474/15/355680-13$15.00/0.
Figures


Similar articles
-
Cholecystokinin selectively activates short axon cells to enhance inhibition of olfactory bulb output neurons.J Physiol. 2018 Jun;596(11):2185-2207. doi: 10.1113/JP275511. Epub 2018 Apr 16. J Physiol. 2018. PMID: 29572837 Free PMC article.
-
The Interglomerular Circuit Potently Inhibits Olfactory Bulb Output Neurons by Both Direct and Indirect Pathways.J Neurosci. 2016 Sep 14;36(37):9604-17. doi: 10.1523/JNEUROSCI.1763-16.2016. J Neurosci. 2016. PMID: 27629712 Free PMC article.
-
Serotonin increases synaptic activity in olfactory bulb glomeruli.J Neurophysiol. 2016 Mar;115(3):1208-19. doi: 10.1152/jn.00847.2015. Epub 2015 Dec 9. J Neurophysiol. 2016. PMID: 26655822 Free PMC article.
-
Dendritic processing within olfactory bulb circuits.Trends Neurosci. 2003 Sep;26(9):501-6. doi: 10.1016/S0166-2236(03)00228-5. Trends Neurosci. 2003. PMID: 12948662 Review.
-
The circuits of the olfactory bulb. The exception as a rule.Anat Rec (Hoboken). 2013 Sep;296(9):1401-12. doi: 10.1002/ar.22732. Epub 2013 Jul 31. Anat Rec (Hoboken). 2013. PMID: 23907743 Review.
Cited by
-
Basal Forebrain Modulation of Olfactory Coding In Vivo.Int J Psychol Res (Medellin). 2023 Oct 10;16(2):62-86. doi: 10.21500/20112084.6486. eCollection 2023 Jul-Dec. Int J Psychol Res (Medellin). 2023. PMID: 38106956 Free PMC article.
-
Quantifying Peripheral Modulation of Olfaction by Trigeminal Agonists.J Neurosci. 2023 Nov 22;43(47):7958-7966. doi: 10.1523/JNEUROSCI.0489-23.2023. J Neurosci. 2023. PMID: 37813571 Free PMC article.
-
Internal Cholinergic Regulation of Learning and Recall in a Model of Olfactory Processing.Front Cell Neurosci. 2016 Nov 8;10:256. doi: 10.3389/fncel.2016.00256. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27877112 Free PMC article.
-
Olfactory bulb acetylcholine release dishabituates odor responses and reinstates odor investigation.Nat Commun. 2018 May 14;9(1):1868. doi: 10.1038/s41467-018-04371-w. Nat Commun. 2018. PMID: 29760390 Free PMC article.
-
Altered Baseline and Nicotine-Mediated Behavioral and Cholinergic Profiles in ChAT-Cre Mouse Lines.J Neurosci. 2018 Feb 28;38(9):2177-2188. doi: 10.1523/JNEUROSCI.1433-17.2018. Epub 2018 Jan 25. J Neurosci. 2018. PMID: 29371319 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

