Role of the sympathetic autonomic nervous system in hypoxic remodeling of the fetal cerebral vasculature
- PMID: 25853949
- PMCID: PMC4391294
- DOI: 10.1097/FJC.0000000000000192
Role of the sympathetic autonomic nervous system in hypoxic remodeling of the fetal cerebral vasculature
Abstract
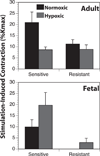
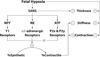
Fetal hypoxia triggers compensatory angiogenesis and remodeling through mechanisms not fully elucidated. In response to hypoxia, hypoxia-inducible factor drives expression of cytokines that exert multiple effects on cerebral structures. Among these, the artery wall is composed of a heterogeneous cell mix and exhibits distinct patterns of cellular differentiation and reactivity. Governing these patterns are the vascular endothelium, smooth muscle (SM), adventitia, sympathetic perivascular nerves (SPN), and the parenchyma. Although an extensive literature details effects of nonneuronal factors on cerebral arteries, the trophic role of perivascular nerves remains unclear. Hypoxia increases sympathetic innervation with subsequent release of norepinephrine (NE), neuropeptide-Y (NPY), and adenosine triphosphate, which exert motor and trophic effects on cerebral arteries and influence dynamic transitions among SM phenotypes. Our data also suggest that the cerebrovasculature reacts very differently to hypoxia in fetuses and adults, and we hypothesize that these differences arise from age-related differences in arterial SM phenotype reactivity and proximity to trophic factors, particularly of neural origin. We provide an integration of recent literature focused on mechanisms by which SPN mediate hypoxic remodeling. Our recent findings suggest that trophic effects of SPN on cerebral arteries accelerate functional maturation through shifts in SM phenotype in an age-dependent manner.
Figures
Similar articles
-
Sympathetic neurogenic Ca2+ signalling in rat arteries: ATP, noradrenaline and neuropeptide Y.Exp Physiol. 2009 Jan;94(1):31-7. doi: 10.1113/expphysiol.2008.043638. Epub 2008 Oct 17. Exp Physiol. 2009. PMID: 18931047 Free PMC article.
-
Neuropeptide Y: a novel mechanism for ischemic angiogenesis.Trends Cardiovasc Med. 2003 Feb;13(2):86-92. doi: 10.1016/s1050-1738(02)00232-3. Trends Cardiovasc Med. 2003. PMID: 12586445 Review.
-
Sympathetically evoked Ca2+ signaling in arterial smooth muscle.Acta Pharmacol Sin. 2006 Dec;27(12):1515-25. doi: 10.1111/j.1745-7254.2006.00465.x. Acta Pharmacol Sin. 2006. PMID: 17112404 Review.
-
Hypoxic regulation of the fetal cerebral circulation.J Appl Physiol (1985). 2006 Feb;100(2):731-8. doi: 10.1152/japplphysiol.00990.2005. J Appl Physiol (1985). 2006. PMID: 16421280 Review.
-
Chronic hypoxia alters prejunctional alpha(2)-receptor function in vascular adrenergic nerves of adult and fetal sheep.Am J Physiol Regul Integr Comp Physiol. 2001 Sep;281(3):R926-34. doi: 10.1152/ajpregu.2001.281.3.R926. Am J Physiol Regul Integr Comp Physiol. 2001. PMID: 11507010
Cited by
-
The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period.Front Neurosci. 2020 Jan 22;13:1452. doi: 10.3389/fnins.2019.01452. eCollection 2019. Front Neurosci. 2020. PMID: 32038147 Free PMC article. Review.
-
Gestational Hypoxia and Developmental Plasticity.Physiol Rev. 2018 Jul 1;98(3):1241-1334. doi: 10.1152/physrev.00043.2017. Physiol Rev. 2018. PMID: 29717932 Free PMC article. Review.
-
Gestational Hypoxia and Programing of Lung Metabolism.Front Physiol. 2019 Nov 29;10:1453. doi: 10.3389/fphys.2019.01453. eCollection 2019. Front Physiol. 2019. PMID: 31849704 Free PMC article. Review.
-
Long-Term Hypoxia Negatively Influences Ca2+ Signaling in Basilar Arterial Myocytes of Fetal and Adult Sheep.Front Physiol. 2022 Jan 18;12:760176. doi: 10.3389/fphys.2021.760176. eCollection 2021. Front Physiol. 2022. PMID: 35115953 Free PMC article.
-
Fetal Cerebrovascular Maturation: Effects of Hypoxia.Semin Pediatr Neurol. 2018 Dec;28:17-28. doi: 10.1016/j.spen.2018.05.003. Epub 2018 Jun 20. Semin Pediatr Neurol. 2018. PMID: 30522724 Free PMC article. Review.
References
-
- Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, Lackritz EM, Lee SK, Mason E, Serazin AC, Walani S, Simpson JL, Lawn JE Born Too Soon preterm prevention analysis g. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013 Jan 19;381(9862):223–234. - PMC - PubMed
-
- Lau C, Ambalavanan N, Chakraborty H, Wingate MS, Carlo WA. Extremely low birth weight and infant mortality rates in the United States. Pediatrics. 2013 May;131(5):855–860. - PubMed
-
- Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, Gladson CL, Beardsley DJ, Murdoch G, Back SA, Tan S. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004 Jan 7;24(1):24–34. - PMC - PubMed
-
- Mohsin M, Bauman AE, Jalaludin B. The influence of antenatal and maternal factors on stillbirths and neonatal deaths in New South Wales, Australia. J Biosoc Sci. 2006 Sep;38(5):643–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

