Proteome readjustments in the apoplastic space of Arabidopsis thaliana ggt1 mutant leaves exposed to UV-B radiation
- PMID: 25852701
- PMCID: PMC4371699
- DOI: 10.3389/fpls.2015.00128
Proteome readjustments in the apoplastic space of Arabidopsis thaliana ggt1 mutant leaves exposed to UV-B radiation
Abstract
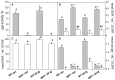
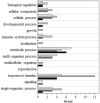
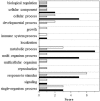
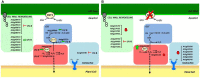
Ultraviolet-B radiation acts as an environmental stimulus, but in high doses it has detrimental effects on plant metabolism. Plasma membranes represent a major target for Reactive Oxygen Species (ROS) generated by this harmful radiation. Oxidative reactions occurring in the apoplastic space are counteracted by antioxidative systems mainly involving ascorbate and, to some extent, glutathione. The occurrence of the latter and its exact role in the extracellular space are not well documented, however. In Arabidopsis thaliana, the gamma-glutamyl transferase isoform (GGT1) bound to the cell wall takes part in the so-called gamma-glutamyl cycle for extracellular glutathione degradation and recovery, and may be implicated in redox sensing and balance. In this work, oxidative conditions were imposed with Ultraviolet-B radiation (UV-B) and studied in redox altered ggt1 mutants. The response of ggt1 knockout Arabidopsis leaves to UV-B radiation was assessed by investigating changes in extracellular glutathione and ascorbate content and their redox state, and in apoplastic protein composition. Our results show that, on UV-B exposure, soluble antioxidants respond to the oxidative conditions in both genotypes. Rearrangements occur in their apoplastic protein composition, suggesting an involvement of Hydrogen Peroxide (H2O2), which may ultimately act as a signal. Other important changes relating to hormonal effects, cell wall remodeling, and redox activities are discussed. We argue that oxidative stress conditions imposed by UV-B and disruption of the gamma-glutamyl cycle result in similar stress-induced responses, to some degree at least. Data are available via ProteomeXchange with identifier PXD001807.
Keywords: apoplast; gamma-glutamyl-transferase; glutathione; iTRAQ labeling; oxidative stress; ultraviolet-B radiation.
Figures
Similar articles
-
Leaf apoplastic proteome composition in UV-B treated Arabidopsis thaliana mutants impaired in extracellular glutathione degradation.Data Brief. 2015 Dec 17;6:368-77. doi: 10.1016/j.dib.2015.12.005. eCollection 2016 Mar. Data Brief. 2015. PMID: 26862584 Free PMC article.
-
Biochemical and quantitative proteomics investigations in Arabidopsis ggt1 mutant leaves reveal a role for the gamma-glutamyl cycle in plant's adaptation to environment.Proteomics. 2013 Jun;13(12-13):2031-45. doi: 10.1002/pmic.201200479. Epub 2013 Jun 7. Proteomics. 2013. PMID: 23661340
-
Apoplastic gamma-glutamyl transferase activity encoded by GGT1 and GGT2 is important for vegetative and generative development.Plant Physiol Biochem. 2017 Jun;115:44-56. doi: 10.1016/j.plaphy.2017.03.007. Epub 2017 Mar 10. Plant Physiol Biochem. 2017. PMID: 28319794
-
Are diverse signalling pathways integrated in the regulation of arabidopsis antioxidant defence gene expression in response to excess excitation energy?Philos Trans R Soc Lond B Biol Sci. 2000 Oct 29;355(1402):1531-40. doi: 10.1098/rstb.2000.0713. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11128006 Free PMC article. Review.
-
Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors.Photochem Photobiol Sci. 2007 Mar;6(3):252-66. doi: 10.1039/b700019g. Epub 2007 Feb 1. Photochem Photobiol Sci. 2007. PMID: 17344961 Review.
Cited by
-
Overview of the Role of Cell Wall DUF642 Proteins in Plant Development.Int J Mol Sci. 2019 Jul 6;20(13):3333. doi: 10.3390/ijms20133333. Int J Mol Sci. 2019. PMID: 31284602 Free PMC article. Review.
-
LSPpred Suite: Tools for Leaderless Secretory Protein Prediction in Plants.Plants (Basel). 2023 Mar 23;12(7):1428. doi: 10.3390/plants12071428. Plants (Basel). 2023. PMID: 37050054 Free PMC article.
-
Regulation of Proline Accumulation and Protein Secretion in Sorghum under Combined Osmotic and Heat Stress.Plants (Basel). 2024 Jul 6;13(13):1874. doi: 10.3390/plants13131874. Plants (Basel). 2024. PMID: 38999714 Free PMC article.
-
Genome-Wide Analyses of Aspartic Proteases on Potato Genome (Solanum tuberosum): Generating New Tools to Improve the Resistance of Plants to Abiotic Stress.Plants (Basel). 2022 Feb 18;11(4):544. doi: 10.3390/plants11040544. Plants (Basel). 2022. PMID: 35214878 Free PMC article.
-
Obligate Biotroph Pathogens of the Genus Albugo Are Better Adapted to Active Host Defense Compared to Niche Competitors.Front Plant Sci. 2016 Jun 20;7:820. doi: 10.3389/fpls.2016.00820. eCollection 2016. Front Plant Sci. 2016. PMID: 27379119 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

