Comparative analysis of human ex vivo-generated platelets vs megakaryocyte-generated platelets in mice: a cautionary tale
- PMID: 25852052
- PMCID: PMC4458801
- DOI: 10.1182/blood-2014-08-593053
Comparative analysis of human ex vivo-generated platelets vs megakaryocyte-generated platelets in mice: a cautionary tale
Abstract
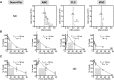
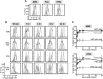
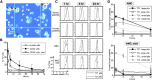
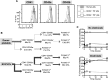
Thrombopoiesis is the process by which megakaryocytes release platelets that circulate as uniform small, disc-shaped anucleate cytoplasmic fragments with critical roles in hemostasis and related biology. The exact mechanism of thrombopoiesis and the maturation pathways of platelets released into the circulation remain incompletely understood. We showed that ex vivo-generated murine megakaryocytes infused into mice release platelets within the pulmonary vasculature. Here we now show that infused human megakaryocytes also release platelets within the lungs of recipient mice. In addition, we observed a population of platelet-like particles (PLPs) in the infusate, which include platelets released during ex vivo growth conditions. By comparing these 2 platelet populations to human donor platelets, we found marked differences: platelets derived from infused megakaryocytes closely resembled infused donor platelets in morphology, size, and function. On the other hand, the PLP was a mixture of nonplatelet cellular fragments and nonuniform-sized, preactivated platelets mostly lacking surface CD42b that were rapidly cleared by macrophages. These data raise a cautionary note for the clinical use of human platelets released under standard ex vivo conditions. In contrast, human platelets released by intrapulmonary-entrapped megakaryocytes appear more physiologic in nature and nearly comparable to donor platelets for clinical application.
© 2015 by The American Society of Hematology.
Figures


Comment in
-
A paradigm shift in platelet transfusion therapy.Blood. 2015 Jun 4;125(23):3523-5. doi: 10.1182/blood-2015-04-640649. Blood. 2015. PMID: 26045593 Free PMC article.
Similar articles
-
Infusion of mature megakaryocytes into mice yields functional platelets.J Clin Invest. 2010 Nov;120(11):3917-22. doi: 10.1172/JCI43326. Epub 2010 Oct 25. J Clin Invest. 2010. PMID: 20972336 Free PMC article.
-
Mechanisms of thrombocytopenia in platelet-type von Willebrand disease.Haematologica. 2019 Jul;104(7):1473-1481. doi: 10.3324/haematol.2018.200378. Epub 2019 Jan 17. Haematologica. 2019. PMID: 30655369 Free PMC article.
-
Studies of infused megakaryocytes into mice support a "catch-and-release" model of pulmonary-centric thrombopoiesis.bioRxiv [Preprint]. 2024 Jun 4:2024.06.04.597316. doi: 10.1101/2024.06.04.597316. bioRxiv. 2024. PMID: 38895231 Free PMC article. Preprint.
-
Induced Pluripotent Stem Cell-Derived Megakaryocytes and Platelets for Disease Modeling and Future Clinical Applications.Arterioscler Thromb Vasc Biol. 2017 Nov;37(11):2007-2013. doi: 10.1161/ATVBAHA.117.309197. Epub 2017 Oct 5. Arterioscler Thromb Vasc Biol. 2017. PMID: 28982668 Free PMC article. Review.
-
Megakaryopoiesis and Platelet Biology: Roles of Transcription Factors and Emerging Clinical Implications.Int J Mol Sci. 2021 Sep 5;22(17):9615. doi: 10.3390/ijms22179615. Int J Mol Sci. 2021. PMID: 34502524 Free PMC article. Review.
Cited by
-
Development of autologous blood cell therapies.Exp Hematol. 2016 Oct;44(10):887-94. doi: 10.1016/j.exphem.2016.06.005. Epub 2016 Jun 21. Exp Hematol. 2016. PMID: 27345108 Free PMC article. Review.
-
Infused factor VIII-expressing platelets or megakaryocytes as a novel therapeutic strategy for hemophilia A.Blood Adv. 2019 May 14;3(9):1368-1378. doi: 10.1182/bloodadvances.2017007914. Blood Adv. 2019. PMID: 31036722 Free PMC article.
-
Current status of blood 'pharming': megakaryoctye transfusions as a source of platelets.Curr Opin Hematol. 2017 Nov;24(6):565-571. doi: 10.1097/MOH.0000000000000378. Curr Opin Hematol. 2017. PMID: 28985194 Free PMC article. Review.
-
Packaging functionally important plasma proteins into the α-granules of human-induced pluripotent stem cell-derived megakaryocytes.J Tissue Eng Regen Med. 2019 Feb;13(2):244-252. doi: 10.1002/term.2785. Epub 2019 Jan 4. J Tissue Eng Regen Med. 2019. PMID: 30556311 Free PMC article.
-
Platelet-targeted gene therapy induces immune tolerance in hemophilia and beyond.J Thromb Haemost. 2024 Jan;22(1):23-34. doi: 10.1016/j.jtha.2023.07.025. Epub 2023 Aug 7. J Thromb Haemost. 2024. PMID: 37558132 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

