In silico pharmacokinetic and molecular docking studies of small molecules derived from Indigofera aspalathoides Vahl targeting receptor tyrosine kinases
- PMID: 25848167
- PMCID: PMC4369682
- DOI: 10.6026/97320630011073
In silico pharmacokinetic and molecular docking studies of small molecules derived from Indigofera aspalathoides Vahl targeting receptor tyrosine kinases
Abstract
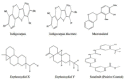
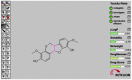
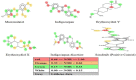
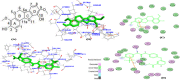
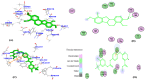
Angiogenesis is the formation of new blood vessels from preexisting vascular network that plays an important role in the tumor growth, invasion and metastasis. Anti-angiogenesis targeting tyrosine kinases such as vascular endothelial growth factor receptor 2 (VEGFR2) and platelet derived growth factor receptor β (PDGFRβ) constitutes a successful target for the treatment of cancer. In this work, molecular docking studies of three bioflavanoid such as indigocarpan, mucronulatol, indigocarpan diacetate and two diterpenes namely erythroxydiol X and Y derived from Indigofera aspalathoides as PDGFRβ and VEGFR2 inhibitors were performed using computational tools. The crystal structures of two target proteins were retrieved from PDB website. Among the five compounds investigated, indigocarpan exhibited potent binding energy ΔG = -7.04 kcal/mol with VEGFR2 and ΔG = -4.82 with PDGFRβ compared to commercially available anti-angiogenic drug sorafenib (positive control). Our results strongly suggested that indigocarpan is a potent angiogenesis inhibitor as ascertained by its potential interaction with VEGFR2 and PDGFRβ. This hypothesis provides a better insight to control metastasis by blocking angiogenesis.
Keywords: Angiogenesis; Autodock; Indigocarpan; Lipinski rules; PDGFRβ; VEGFR2.
Figures
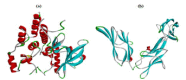

Similar articles
-
Computational insights into the identification of a potent matrix metalloproteinase inhibitor from Indigofera aspalathoides to control cancer metastasis.3 Biotech. 2021 May;11(5):206. doi: 10.1007/s13205-021-02731-w. Epub 2021 Apr 7. 3 Biotech. 2021. PMID: 33927994 Free PMC article.
-
Antioxidant and antiproliferative activity of indigocarpan, a pterocarpan from Indigofera aspalathoides.J Pharm Pharmacol. 2016 Oct;68(10):1331-9. doi: 10.1111/jphp.12609. Epub 2016 Jul 27. J Pharm Pharmacol. 2016. PMID: 27464528
-
Identification of tyrosine kinase inhibitors from Panax bipinnatifidus and Panax pseudoginseng for RTK-HER2 and VEGFR2 receptors, by in silico approach.Mol Divers. 2022 Aug;26(4):1933-1955. doi: 10.1007/s11030-021-10304-5. Epub 2021 Sep 23. Mol Divers. 2022. PMID: 34554395
-
Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy.Curr Med Chem Anticancer Agents. 2003 Mar;3(2):95-117. doi: 10.2174/1568011033353452. Curr Med Chem Anticancer Agents. 2003. PMID: 12678905 Review.
-
Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth.Prog Biophys Mol Biol. 2013 Nov;113(2):333-54. doi: 10.1016/j.pbiomolbio.2013.10.001. Epub 2013 Oct 15. Prog Biophys Mol Biol. 2013. PMID: 24139944 Review.
Cited by
-
Promising phytochemicals of traditional Indian herbal steam inhalation therapy to combat COVID-19 - An in silico study.Food Chem Toxicol. 2021 Feb;148:111966. doi: 10.1016/j.fct.2020.111966. Epub 2021 Jan 4. Food Chem Toxicol. 2021. PMID: 33412235 Free PMC article.
-
Valorization of hydro-distillate of fruit peels of Citrus paradisi macfad. Cultivar. Foster: Chemical profiling, antioxidant evaluation and in vitro and in silico enzyme inhibition studies.Heliyon. 2024 Aug 21;10(17):e36226. doi: 10.1016/j.heliyon.2024.e36226. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39281520 Free PMC article.
-
Chromatographic analysis of selected phytosterols from Cyathea and their characterization by in silico docking to potential therapeutic targets.Curr Res Toxicol. 2023 Jul 27;5:100115. doi: 10.1016/j.crtox.2023.100115. eCollection 2023. Curr Res Toxicol. 2023. PMID: 37575338 Free PMC article.
-
Identification, ADMET evaluation and molecular docking analysis of Phytosterols from Banaba (Lagerstroemia speciosa (L.)Pers) seed extract against breast cancer.In Silico Pharmacol. 2021 Jul 19;9(1):43. doi: 10.1007/s40203-021-00104-y. eCollection 2021. In Silico Pharmacol. 2021. PMID: 34367875 Free PMC article.
-
Structural and Biofunctional Insights into the Cyclo(Pro-Pro-Phe-Phe-) Scaffold from Experimental and In Silico Studies: Melanoma and Beyond.Int J Mol Sci. 2022 Jun 28;23(13):7173. doi: 10.3390/ijms23137173. Int J Mol Sci. 2022. PMID: 35806175 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
