Aspergillus nidulans Ambient pH Signaling Does Not Require Endocytosis
- PMID: 25841020
- PMCID: PMC4452571
- DOI: 10.1128/EC.00031-15
Aspergillus nidulans Ambient pH Signaling Does Not Require Endocytosis
Abstract
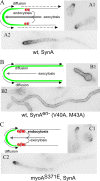
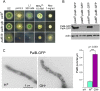
Aspergillus nidulans (Pal) ambient pH signaling takes place in cortical structures containing components of the ESCRT pathway, which are hijacked by the alkaline pH-activated, ubiquitin-modified version of the arrestin-like protein PalF and taken to the plasma membrane. There, ESCRTs scaffold the assembly of dedicated Pal proteins acting downstream. The molecular details of this pathway, which results in the two-step proteolytic processing of the transcription factor PacC, have received considerable attention due to the key role that it plays in fungal pathogenicity. While current evidence strongly indicates that the pH signaling role of ESCRT complexes is limited to plasma membrane-associated structures where PacC proteolysis would take place, the localization of the PalB protease, which almost certainly catalyzes the first and only pH-regulated proteolytic step, had not been investigated. In view of ESCRT participation, this formally leaves open the possibility that PalB activation requires endocytic internalization. As endocytosis is essential for hyphal growth, nonlethal endocytic mutations are predicted to cause an incomplete block. We used a SynA internalization assay to measure the extent to which any given mutation prevents endocytosis. We show that none of the tested mutations impairing endocytosis to different degrees, including slaB1, conditionally causing a complete block, have any effect on the activation of the pathway. We further show that PalB, like PalA and PalC, localizes to cortical structures in an alkaline pH-dependent manner. Therefore, signaling through the Pal pathway does not involve endocytosis.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Liaison alcaline: Pals entice non-endosomal ESCRTs to the plasma membrane for pH signaling.Curr Opin Microbiol. 2014 Dec;22:49-59. doi: 10.1016/j.mib.2014.09.005. Curr Opin Microbiol. 2014. PMID: 25460796 Review.
-
An ordered pathway for the assembly of fungal ESCRT-containing ambient pH signalling complexes at the plasma membrane.J Cell Sci. 2012 Apr 1;125(Pt 7):1784-95. doi: 10.1242/jcs.098897. Epub 2012 Feb 17. J Cell Sci. 2012. PMID: 22344261 Free PMC article.
-
Physiological involvement in pH signaling of Vps24-mediated recruitment of Aspergillus PalB cysteine protease to ESCRT-III.J Biol Chem. 2009 Feb 13;284(7):4404-12. doi: 10.1074/jbc.M808645200. Epub 2008 Dec 3. J Biol Chem. 2009. PMID: 19056728 Free PMC article.
-
PalC, one of two Bro1 domain proteins in the fungal pH signalling pathway, localizes to cortical structures and binds Vps32.Traffic. 2007 Oct;8(10):1346-64. doi: 10.1111/j.1600-0854.2007.00620.x. Epub 2007 Aug 13. Traffic. 2007. PMID: 17696968 Free PMC article.
-
Ambient pH gene regulation in fungi: making connections.Trends Microbiol. 2008 Jun;16(6):291-300. doi: 10.1016/j.tim.2008.03.006. Epub 2008 May 3. Trends Microbiol. 2008. PMID: 18457952 Review.
Cited by
-
Genomic footprints related with adaptation and fumonisins production in Fusarium proliferatum.Front Microbiol. 2022 Sep 21;13:1004454. doi: 10.3389/fmicb.2022.1004454. eCollection 2022. Front Microbiol. 2022. PMID: 36212817 Free PMC article.
-
Molecular Components of the Neurospora crassa pH Signaling Pathway and Their Regulation by pH and the PAC-3 Transcription Factor.PLoS One. 2016 Aug 24;11(8):e0161659. doi: 10.1371/journal.pone.0161659. eCollection 2016. PLoS One. 2016. PMID: 27557053 Free PMC article.
-
Refining the pH response in Aspergillus nidulans: a modulatory triad involving PacX, a novel zinc binuclear cluster protein.Mol Microbiol. 2015 Dec;98(6):1051-72. doi: 10.1111/mmi.13173. Epub 2015 Oct 16. Mol Microbiol. 2015. PMID: 26303777 Free PMC article.
-
Alkaliphilic/Alkali-Tolerant Fungi: Molecular, Biochemical, and Biotechnological Aspects.J Fungi (Basel). 2023 Jun 9;9(6):652. doi: 10.3390/jof9060652. J Fungi (Basel). 2023. PMID: 37367588 Free PMC article. Review.
-
Interconnections between the Cation/Alkaline pH-Responsive Slt and the Ambient pH Response of PacC/Pal Pathways in Aspergillus nidulans.Cells. 2024 Apr 8;13(7):651. doi: 10.3390/cells13070651. Cells. 2024. PMID: 38607089 Free PMC article.
References
-
- Calcagno-Pizarelli AM, Negrete-Urtasun S, Denison SH, Rudnicka JD, Bussink HJ, Munera-Huertas T, Stanton L, Hervás-Aguilar A, Espeso EA, Tilburn J, Arst HN Jr, Peñalva MA. 2007. Establishment of the ambient pH signaling complex in Aspergillus nidulans: PalI assists plasma membrane localization of PalH. Eukaryot Cell 6:2365–2375. doi:10.1128/EC.00275-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

