Induction of apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated pathways by plumbagin in human prostate cancer cells
- PMID: 25834399
- PMCID: PMC4366042
- DOI: 10.2147/DDDT.S75976
Induction of apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated pathways by plumbagin in human prostate cancer cells
Retraction in
-
Induction of Apoptosis and Autophagy via sirtuin1- and PI3K/Akt/mTOR-Mediated Pathways by Plumbagin in Human Prostate Cancer Cells [Retraction].Drug Des Devel Ther. 2022 Jul 29;16:2437-2438. doi: 10.2147/DDDT.S383775. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35937567 Free PMC article.
Abstract
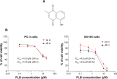
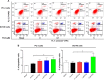
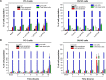
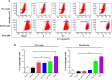
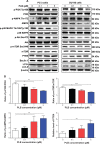
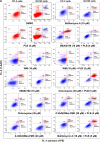
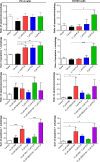
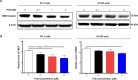
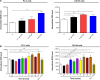
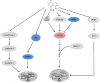
Plumbagin (PLB) has been shown to have anticancer activities in animal models, but the role of PLB in prostate cancer treatment is unclear. This study aimed to investigate the effects of PLB on apoptosis and autophagy and the underlying mechanisms in human prostate cancer cell lines PC-3 and DU145. Our study has shown that PLB had potent pro-apoptotic and pro-autophagic effects on PC-3 and DU145 cells. PLB induced mitochondria-mediated apoptosis and autophagy in concentration- and time-dependent manners in both PC-3 and DU145 cells. PLB induced inhibition of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) and p38 mitogen-activated protein kinase (MAPK) pathways and activation of 5'-AMP-dependent kinase (AMPK) as indicated by their altered phosphorylation, contributing to the pro-autophagic activity of PLB. Modulation of autophagy altered basal and PLB-induced apoptosis in both cell lines. Furthermore, PLB downregulated sirtuin 1 (Sirt1), and inhibition of Sirt1 enhanced autophagy, whereas the induction of Sirt1 abolished PLB-induced autophagy in PC-3 and DU145 cells. In addition, PLB downregulated pre-B cell colony-enhancing factor/visfatin, and the inhibition of pre-B cell colony-enhancing factor/visfatin significantly enhanced basal and PLB-induced apoptosis and autophagy in both cell lines. Moreover, reduction of intracellular reactive oxygen species (ROS) level attenuated the apoptosis- and autophagy-inducing effects of PLB on both PC-3 and DU145 cells. These findings indicate that PLB promotes apoptosis and autophagy in prostate cancer cells via Sirt1- and PI3K/Akt/mTOR-mediated pathways with contribution from AMPK-, p38 MAPK-, visfatin-, and ROS-associated pathways.
Keywords: AMPK; DU145; PC-3; ROS; visfatin.
Figures



















Similar articles
-
Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK- and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells.Drug Des Devel Ther. 2015 Mar 16;9:1601-26. doi: 10.2147/DDDT.S76057. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25834400 Free PMC article.
-
Plumbagin induces cell cycle arrest and autophagy and suppresses epithelial to mesenchymal transition involving PI3K/Akt/mTOR-mediated pathway in human pancreatic cancer cells.Drug Des Devel Ther. 2015 Jan 17;9:537-60. doi: 10.2147/DDDT.S73689. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25632222 Free PMC article.
-
Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway.Drug Des Devel Ther. 2015 Mar 12;9:1555-84. doi: 10.2147/DDDT.S74197. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25792811 Free PMC article.
-
Autophagy: novel applications of nonsteroidal anti-inflammatory drugs for primary cancer.Cancer Med. 2018 Feb;7(2):471-484. doi: 10.1002/cam4.1287. Epub 2017 Dec 28. Cancer Med. 2018. PMID: 29282893 Free PMC article. Review.
-
Antidepressant Drug Sertraline against Human Cancer Cells.Biomolecules. 2022 Oct 19;12(10):1513. doi: 10.3390/biom12101513. Biomolecules. 2022. PMID: 36291722 Free PMC article. Review.
Cited by
-
Gene Expression Regulation and the Signal Transduction of Programmed Cell Death.Curr Issues Mol Biol. 2024 Sep 16;46(9):10264-10298. doi: 10.3390/cimb46090612. Curr Issues Mol Biol. 2024. PMID: 39329964 Free PMC article. Review.
-
Proteasome Inhibitor-Induced IκB/NF-κB Activation is Mediated by Nrf2-Dependent Light Chain 3B Induction in Lung Cancer Cells.Mol Cells. 2018 Dec 31;41(12):1008-1015. doi: 10.14348/molcells.2018.0277. Epub 2018 Nov 6. Mol Cells. 2018. PMID: 30396235 Free PMC article.
-
Knockdown of SIRT1 Suppresses Bladder Cancer Cell Proliferation and Migration and Induces Cell Cycle Arrest and Antioxidant Response through FOXO3a-Mediated Pathways.Biomed Res Int. 2017;2017:3781904. doi: 10.1155/2017/3781904. Epub 2017 Sep 25. Biomed Res Int. 2017. PMID: 29147649 Free PMC article.
-
MiR-345 suppresses proliferation, migration and invasion by targeting Smad1 in human prostate cancer.J Cancer Res Clin Oncol. 2016 Jan;142(1):213-24. doi: 10.1007/s00432-015-2016-0. Epub 2015 Jul 31. J Cancer Res Clin Oncol. 2016. PMID: 26227059
-
HDACs and HDAC Inhibitors in Cancer Development and Therapy.Cold Spring Harb Perspect Med. 2016 Oct 3;6(10):a026831. doi: 10.1101/cshperspect.a026831. Cold Spring Harb Perspect Med. 2016. PMID: 27599530 Free PMC article. Review.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. - PubMed
-
- Ahmed HU. Prostate cancer: time for active surveillance of intermediate-risk disease? Nat Rev Urol. 2013;10(1):6–8. - PubMed
-
- American Cancer Society . Global Cancer Facts and Figures. Atlanta: American Cancer Society; 2011.
-
- U.S., Cancer Statistics Working Group, United States cancer statistics: 1999–2011 incidence and mortality web-based report. Centers for Disease Control and Prevention and National Cancer Institute; Atlanta, GA: 2014.
-
- Cancer mortality statistics: Cancer mortality for common cancers. London: Cancer Research UK; [Accessed November 6, 2014]. [updated September 15, 2014]. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/mortality/cancer....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

