Opiorphin-dependent upregulation of CD73 (a key enzyme in the adenosine signaling pathway) in corporal smooth muscle cells exposed to hypoxic conditions and in corporal tissue in pre-priapic sickle cell mice
- PMID: 25833166
- PMCID: PMC4504813
- DOI: 10.1038/ijir.2015.5
Opiorphin-dependent upregulation of CD73 (a key enzyme in the adenosine signaling pathway) in corporal smooth muscle cells exposed to hypoxic conditions and in corporal tissue in pre-priapic sickle cell mice
Abstract
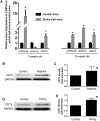
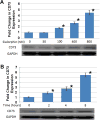
The precise molecular mechanisms underlying priapism associated with sickle cell disease remain to be defined. However, there is increasing evidence that upregulated activity of the opiorphin and adenosine pathways in corporal tissue, resulting in heighted relaxation of smooth muscle, have an important role in development of priapism. A key enzyme in the adenosine pathway is CD73, an ecto-5'-nucleotidase (5'-ribonucleotide phosphohydrolase; EC 3.1.3.5) which catalyzes the conversion of adenosine mononucleotides to adenosine. In the present study we investigated how sickle cell disease and hypoxia regulate the interplay between opiorphin and CD73. In the corpora of sickle cell mice we observed significantly elevated expression of both the mouse opiorphin homolog mSmr3a (14-fold) and CD73 (2.2-fold) relative to non-sickle cell controls at a life stage before the exhibition of priapism. Sickle cell disease has a pronounced hypoxic component, therefore we determined if CD73 was also modulated in in vitro corporal smooth muscle (CSM) models of hypoxia. Hypoxia significantly increased CD73 protein and mRNA expression by 1.5-fold and 2-fold, respectively. We previously demonstrated that expression of another component of the adenosine signaling pathway, the adensosine 2B receptor, can be regulated by sialorphin (the rat opiorphin homolologue), and we demonstrate that sialorphin also regulates CD73 expression in a dose- and time-dependent fashion. Using siRNA to knockdown sialorphin mRNA expression in CSM cells in vitro, we demonstrate that the hypoxic upregulation of CD73 is dependent on the upregulation of sialorphin. Overall, our data provide further evidence to support a role for opiorphin in CSM in regulating the cellular response to hypoxia or sickle cell disease by activating smooth muscle relaxant pathways.
Figures
Similar articles
-
Opiorphin is a master regulator of the hypoxic response in corporal smooth muscle cells.FASEB J. 2014 Aug;28(8):3633-44. doi: 10.1096/fj.13-248708. Epub 2014 May 6. FASEB J. 2014. PMID: 24803544 Free PMC article.
-
The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway.Am J Physiol Cell Physiol. 2009 Oct;297(4):C916-27. doi: 10.1152/ajpcell.00656.2008. Epub 2009 Aug 5. Am J Physiol Cell Physiol. 2009. PMID: 19657052 Free PMC article.
-
The opiorphin gene (ProL1) and its homologues function in erectile physiology.BJU Int. 2008 Sep;102(6):736-40. doi: 10.1111/j.1464-410X.2008.07631.x. Epub 2008 Apr 10. BJU Int. 2008. PMID: 18410445 Free PMC article.
-
New insights into the pathophysiology of sickle cell disease-associated priapism.J Sex Med. 2012 Jan;9(1):79-87. doi: 10.1111/j.1743-6109.2011.02288.x. Epub 2011 May 6. J Sex Med. 2012. PMID: 21554553 Review.
-
The role of opiorphins (endogenous neutral endopeptidase inhibitors) in urogenital smooth muscle biology.J Sex Med. 2009 Mar;6 Suppl 3(Suppl 3):286-91. doi: 10.1111/j.1743-6109.2008.01186.x. J Sex Med. 2009. PMID: 19267851 Free PMC article. Review.
Cited by
-
Hypoxia-enhanced adhesion of red blood cells in microscale flow.Microcirculation. 2017 Jul;24(5):10.1111/micc.12374. doi: 10.1111/micc.12374. Microcirculation. 2017. PMID: 28387057 Free PMC article.
-
Advances in the understanding of priapism.Transl Androl Urol. 2017 Apr;6(2):199-206. doi: 10.21037/tau.2017.01.18. Transl Androl Urol. 2017. PMID: 28540227 Free PMC article. Review.
-
Purinergic Signalling: Therapeutic Developments.Front Pharmacol. 2017 Sep 25;8:661. doi: 10.3389/fphar.2017.00661. eCollection 2017. Front Pharmacol. 2017. PMID: 28993732 Free PMC article. Review.
-
PROL1 is essential for xenograft tumor development in mice injected with the human prostate cancer cell-line, LNCaP, and modulates cell migration and invasion.J Mens Health. 2022 Feb;18(2):044. doi: 10.31083/jomh.2021.131. Epub 2021 Oct 14. J Mens Health. 2022. PMID: 35547856 Free PMC article.
-
Cancer Stem Cells: Emergent Nature of Tumor Emergency.Front Genet. 2018 Nov 16;9:544. doi: 10.3389/fgene.2018.00544. eCollection 2018. Front Genet. 2018. PMID: 30505319 Free PMC article.
References
-
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med. 1980;140(11):1434–7. - PubMed
-
- Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003;170(2 Pt 2):S6–13. discussion S-4. - PubMed
-
- Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257(5068):401–3. - PubMed
-
- Dai Y, Zhang Y, Phatarpekar P, Mi T, Zhang H, Blackburn MR, et al. Adenosine signaling, priapism and novel therapies. J Sex Med. 2009;6(Suppl 3):292–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

