Morphogenesis of Endoplasmic Reticulum Membrane-Invaginated Vesicles during Beet Black Scorch Virus Infection: Role of Auxiliary Replication Protein and New Implications of Three-Dimensional Architecture
- PMID: 25833056
- PMCID: PMC4474299
- DOI: 10.1128/JVI.00401-15
Morphogenesis of Endoplasmic Reticulum Membrane-Invaginated Vesicles during Beet Black Scorch Virus Infection: Role of Auxiliary Replication Protein and New Implications of Three-Dimensional Architecture
Abstract
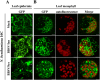
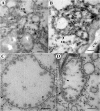
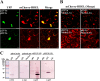
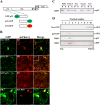
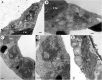
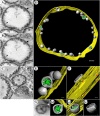
All well-characterized positive-strand RNA viruses[(+)RNA viruses] induce the formation of host membrane-bound viral replication complexes (VRCs), yet the underlying mechanism and machinery for VRC formation remain elusive. We report here the biogenesis and topology of the Beet black scorch virus (BBSV) replication complex. Distinct cytopathological changes typical of endoplasmic reticulum (ER) aggregation and vesiculation were observed in BBSV-infected Nicotiana benthamiana cells. Immunogold labeling of the auxiliary replication protein p23 and double-stranded RNA (dsRNA) revealed that the ER-derived membranous spherules provide the site for BBSV replication. Further studies indicated that p23 plays a crucial role in mediating the ER rearrangement. Three-dimensional electron tomographic analysis revealed the formation of multiple ER-originated vesicle packets. Each vesicle packet enclosed a few to hundreds of independent spherules that were invaginations of the ER membranes into the lumen. Strikingly, these vesicle packets were connected to each other via tubules, a rearrangement event that is rare among other virus-induced membrane reorganizations. Fibrillar contents within the spherules were also reconstructed by electron tomography, which showed diverse structures. Our results provide the first, to our knowledge, three-dimensional ultrastructural analysis of membrane-bound VRCs of a plant (+)RNA virus and should help to achieve a better mechanistic understanding of the organization and microenvironment of plant (+)RNA virus replication complexes.
Importance: Assembly of virus replication complexes for all known positive-strand RNA viruses depends on the extensive remodeling of host intracellular membranes. Beet black scorch virus, a necrovirus in the family Tombusviridae, invaginates the endoplasmic reticulum (ER) membranes to form spherules in infected cells. Double-stranded RNAs, the viral replication intermediate, and the viral auxiliary replication protein p23 are all localized within such viral spherules, indicating that these are the sites for generating progeny viral RNAs. Furthermore, the BBSV p23 protein could to some extent reorganize the ER when transiently expressed in N. benthamiana. Electron tomographic analysis resolves the three-dimensional (3D) architecture of such spherules, which are connected to the cytoplasm via a neck-like structure. Strikingly, different numbers of spherules are enclosed in ER-originated vesicle packets that are connected to each other via tubule-like structures. Our results have significant implications for further understanding the mechanisms underlying the replication of positive-strand RNA viruses.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Infectious bronchitis virus generates spherules from zippered endoplasmic reticulum membranes.mBio. 2013 Oct 22;4(5):e00801-13. doi: 10.1128/mBio.00801-13. mBio. 2013. PMID: 24149513 Free PMC article.
-
RETICULON-LIKE PROTEIN B2 is a proviral factor co-opted for the biogenesis of viral replication organelles in plants.Plant Cell. 2023 Aug 2;35(8):3127-3151. doi: 10.1093/plcell/koad146. Plant Cell. 2023. PMID: 37216674 Free PMC article.
-
ADP ribosylation factor 1 plays an essential role in the replication of a plant RNA virus.J Virol. 2013 Jan;87(1):163-76. doi: 10.1128/JVI.02383-12. Epub 2012 Oct 24. J Virol. 2013. PMID: 23097452 Free PMC article.
-
Bromovirus-induced remodeling of host membranes during viral RNA replication.Curr Opin Virol. 2014 Dec;9:104-10. doi: 10.1016/j.coviro.2014.09.018. Epub 2014 Oct 16. Curr Opin Virol. 2014. PMID: 25462441 Review.
-
Compartmentalized replication organelle of flavivirus at the ER and the factors involved.Cell Mol Life Sci. 2021 Jun;78(11):4939-4954. doi: 10.1007/s00018-021-03834-6. Epub 2021 Apr 12. Cell Mol Life Sci. 2021. PMID: 33846827 Free PMC article. Review.
Cited by
-
Function, Architecture, and Biogenesis of Reovirus Replication Neoorganelles.Viruses. 2019 Mar 21;11(3):288. doi: 10.3390/v11030288. Viruses. 2019. PMID: 30901959 Free PMC article. Review.
-
An Amphipathic Alpha-Helix Domain from Poliovirus 2C Protein Tubulate Lipid Vesicles.Viruses. 2020 Dec 18;12(12):1466. doi: 10.3390/v12121466. Viruses. 2020. PMID: 33353144 Free PMC article.
-
Biogenesis and architecture of arterivirus replication organelles.Virus Res. 2016 Jul 15;220:70-90. doi: 10.1016/j.virusres.2016.04.001. Epub 2016 Apr 9. Virus Res. 2016. PMID: 27071852 Free PMC article. Review.
-
Electron microscopy for imaging organelles in plants and algae.Plant Physiol. 2022 Feb 4;188(2):713-725. doi: 10.1093/plphys/kiab449. Plant Physiol. 2022. PMID: 35235662 Free PMC article.
-
A Novel Mechanism Underlying the Innate Immune Response Induction upon Viral-Dependent Replication of Host Cell mRNA: A Mistake of +sRNA Viruses' Replicases.Front Cell Infect Microbiol. 2017 Jan 20;7:5. doi: 10.3389/fcimb.2017.00005. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28164038 Free PMC article. Review.
References
-
- Cottam E, Pierini R, Roberts R, Wileman T. 2009. Origins of membrane vesicles generated during replication of positive-strand RNA viruses. Future Virol 4:473–485. doi:10.2217/fvl.09.26. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

