Decreased vesicular storage and aldehyde dehydrogenase activity in multiple system atrophy
- PMID: 25829070
- PMCID: PMC4441851
- DOI: 10.1016/j.parkreldis.2015.03.006
Decreased vesicular storage and aldehyde dehydrogenase activity in multiple system atrophy
Abstract
Background: Parkinson disease (PD) and multiple system atrophy (MSA) share some neuropathologic features (nigrostriatal dopaminergic lesion, alpha-synuclein deposition) but not others (Lewy bodies in PD, glial cytoplasmic inclusions in MSA). In PD evidence has accrued for a vesicular storage defect and decreased aldehyde dehydrogenase (ALDH) activity in residual dopaminergic terminals, resulting in accumulation of the toxic dopamine (DA) metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL). In this study we asked whether MSA entails a similar abnormal neurochemical pattern.
Methods: DA and its main neuronal metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), norepinephrine (NE) and its main neuronal metabolite 3,4-dihydroxyphenylglycol (DHPG), the catecholamine precursor DOPA, and DOPAL were measured in striatal and frontal cortical tissue from patients with pathologically proven end-stage MSA (N = 15), sporadic PD (N = 17), and control subjects (N = 18).
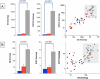
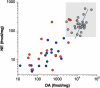
Results: Compared to the control group, the MSA and PD groups had similarly decreased putamen DA (by 96% and 93%, p < 0.0001), DOPAC (97% and 95%, p < 0.0001), NE (91% and 74%, p < 0.0001), and DHPG (81% and 74%, p < 0.0001). In the MSA and PD groups, ratios of DOPAL:DA were 2.3 and 3.5 times control and DHPG:NE 3.1 and 2.6 times control, while DOPAC:DOPAL ratios were decreased by 61% and 74%. In both diseases cortical NE and DHPG were decreased, while DA and DOPAC were not.
Conclusions: MSA and PD entail a catecholamine metabolic profile indicating impaired vesicular storage, decreased ALDH activity, and DOPAL buildup, which might be part of a common pathway in catecholamine neuronal death. Targeting this pathway by interfering with catecholaldehyde production or effects constitutes a novel treatment approach.
Keywords: DOPAL; Dopamine; Multiple system atrophy; Norepinephrine; Parkinson disease; Putamen.
Published by Elsevier Ltd.
Figures

Similar articles
-
Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson's disease.J Neurochem. 2013 Sep;126(5):591-603. doi: 10.1111/jnc.12345. Epub 2013 Jul 22. J Neurochem. 2013. PMID: 23786406 Free PMC article.
-
Plasma biomarkers of decreased vesicular storage distinguish Parkinson disease with orthostatic hypotension from the parkinsonian form of multiple system atrophy.Clin Auton Res. 2015 Feb;25(1):61-7. doi: 10.1007/s10286-015-0268-z. Epub 2015 Feb 1. Clin Auton Res. 2015. PMID: 25638582 Free PMC article.
-
Rotenone decreases intracellular aldehyde dehydrogenase activity: implications for the pathogenesis of Parkinson's disease.J Neurochem. 2015 Apr;133(1):14-25. doi: 10.1111/jnc.13042. Epub 2015 Feb 25. J Neurochem. 2015. PMID: 25645689 Free PMC article.
-
The heart of PD: Lewy body diseases as neurocardiologic disorders.Brain Res. 2019 Jan 1;1702:74-84. doi: 10.1016/j.brainres.2017.09.033. Epub 2017 Oct 10. Brain Res. 2019. PMID: 29030055 Free PMC article. Review.
-
The "Sick-but-not-Dead" Phenomenon Applied to Catecholamine Deficiency in Neurodegenerative Diseases.Semin Neurol. 2020 Oct;40(5):502-514. doi: 10.1055/s-0040-1713874. Epub 2020 Sep 9. Semin Neurol. 2020. PMID: 32906170 Free PMC article. Review.
Cited by
-
Elevated cerebrospinal fluid ratios of cysteinyl-dopamine/3,4-dihydroxyphenylacetic acid in parkinsonian synucleinopathies.Parkinsonism Relat Disord. 2016 Oct;31:79-86. doi: 10.1016/j.parkreldis.2016.07.009. Epub 2016 Jul 20. Parkinsonism Relat Disord. 2016. PMID: 27474472 Free PMC article.
-
3,4-Dihydroxyphenylacetaldehyde-Induced Protein Modifications and Their Mitigation by N-Acetylcysteine.J Pharmacol Exp Ther. 2018 Jul;366(1):113-124. doi: 10.1124/jpet.118.248492. Epub 2018 Apr 26. J Pharmacol Exp Ther. 2018. PMID: 29700232 Free PMC article.
-
Linking Stress, Catecholamine Autotoxicity, and Allostatic Load with Neurodegenerative Diseases: A Focused Review in Memory of Richard Kvetnansky.Cell Mol Neurobiol. 2018 Jan;38(1):13-24. doi: 10.1007/s10571-017-0497-x. Epub 2017 May 9. Cell Mol Neurobiol. 2018. PMID: 28488009 Free PMC article. Review.
-
DOPAL is transmissible to and oligomerizes alpha-synuclein in human glial cells.Auton Neurosci. 2016 Jan;194:46-51. doi: 10.1016/j.autneu.2015.12.008. Epub 2015 Dec 31. Auton Neurosci. 2016. PMID: 26777075 Free PMC article.
-
Oxidative Transformations of 3,4-Dihydroxyphenylacetaldehyde Generate Potential Reactive Intermediates as Causative Agents for Its Neurotoxicity.Int J Mol Sci. 2021 Oct 29;22(21):11751. doi: 10.3390/ijms222111751. Int J Mol Sci. 2021. PMID: 34769179 Free PMC article.
References
-
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–22. - PubMed
-
- Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249:180–2. - PubMed
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. - PubMed
-
- Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, et al. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain. 2010;133:172–88. - PubMed
-
- Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism--a prospective study. Can J Neurol Sci. 1991;18:275–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

