Intracellular EP2 prostanoid receptor promotes cancer-related phenotypes in PC3 cells
- PMID: 25828575
- PMCID: PMC11113933
- DOI: 10.1007/s00018-015-1891-5
Intracellular EP2 prostanoid receptor promotes cancer-related phenotypes in PC3 cells
Abstract
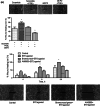
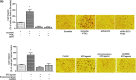
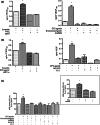
Prostaglandin E2 (PGE2) and hypoxia-inducible factor-1α (HIF-1α) affect many mechanisms that have been involved in the pathogenesis of prostate cancer (PC). HIF-1α, which is up-regulated by PGE2 in LNCaP cells and PC3 cells, has been shown to contribute to metastasis and chemo-resistance of castrate-resistant PC (a lethal form of PC) and to promote in PC cells migration, invasion, angiogenesis and chemoresistance. The selective blockade of PGE2-EP2 signaling pathway in PC3 cells results in inhibition of cancer cell proliferation and invasion. PGE2 affects many mechanisms that have been shown to play a role in carcinogenesis such as proliferation, apoptosis, migration, invasion and angiogenesis. Recently, we have found in PC3 cells that most of these PGE2-induced cancer-related features are due to intracellular PGE2 (iPGE2). Here, we aimed to study in PC3 cells the role of iPGE2-intracellular EP2 (iEP2)-HIF-1α signaling in several events linked to PC progression using an experimental approach involving pharmacological inhibition of the prostaglandin uptake transporter and EGFR and pharmacological and genetic modulation of EP2 receptor and HIF-1α. We found that iPGE2 increases HIF-1α expression through iEP2-dependent EGFR transactivation and that inhibition of any of the axis iEP2-EGFR-HIF-1α in cells treated with PGE2 or EP2 agonist results in prevention of the increase in PC3 cell proliferation, adhesion, migration, invasion and angiogenesis in vitro. Of note, PGE2 induced EP2 antagonist-sensitive DNA synthesis in nuclei isolated from PC3 cells, which indicates that they have functional EP2 receptors. These results suggest that PGE2-EP2 dependent intracrine mechanisms involving EGFR and HIF-1α play a role in PC.
Figures








Similar articles
-
PROSTAGLANDIN E2 stimulates cancer-related phenotypes in prostate cancer PC3 cells through cyclooxygenase-2.J Cell Physiol. 2019 May;234(5):7548-7559. doi: 10.1002/jcp.27515. Epub 2018 Oct 26. J Cell Physiol. 2019. PMID: 30367494
-
Role of intracellular prostaglandin E₂ in cancer-related phenotypes in PC3 cells.Int J Biochem Cell Biol. 2015 Feb;59:52-61. doi: 10.1016/j.biocel.2014.11.004. Epub 2014 Nov 11. Int J Biochem Cell Biol. 2015. PMID: 25462156
-
Intracrine prostaglandin E2 pro-tumoral actions in prostate epithelial cells originate from non-canonical pathways.J Cell Physiol. 2018 Apr;233(4):3590-3602. doi: 10.1002/jcp.26220. Epub 2017 Nov 20. J Cell Physiol. 2018. PMID: 29154474
-
Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha.Int J Urol. 2007 Nov;14(11):1034-9. doi: 10.1111/j.1442-2042.2007.01866.x. Int J Urol. 2007. PMID: 17956532
-
Silymarin suppresses the PGE2 -induced cell migration through inhibition of EP2 activation; G protein-dependent PKA-CREB and G protein-independent Src-STAT3 signal pathways.Mol Carcinog. 2015 Mar;54(3):216-28. doi: 10.1002/mc.22092. Epub 2013 Oct 11. Mol Carcinog. 2015. PMID: 24127286
Cited by
-
Novel water-soluble lignin derivative BP-Cx-1: identification of components and screening of potential targets in silico and in vitro.Oncotarget. 2018 Apr 6;9(26):18578-18593. doi: 10.18632/oncotarget.24990. eCollection 2018 Apr 6. Oncotarget. 2018. PMID: 29719628 Free PMC article.
-
Cooperation between Prostaglandin E2 and Epidermal Growth Factor Receptor in Cancer Progression: A Dual Target for Cancer Therapy.Cancers (Basel). 2023 Apr 19;15(8):2374. doi: 10.3390/cancers15082374. Cancers (Basel). 2023. PMID: 37190301 Free PMC article. Review.
-
Caprylic acid (C8:0) promotes bone metastasis of prostate cancer by dysregulated adipo-osteogenic balance in bone marrow.Cancer Sci. 2020 Oct;111(10):3600-3612. doi: 10.1111/cas.14606. Epub 2020 Aug 28. Cancer Sci. 2020. PMID: 32770813 Free PMC article.
-
The role of HIF in angiogenesis, lymphangiogenesis, and tumor microenvironment in urological cancers.Mol Biol Rep. 2023 Dec 12;51(1):14. doi: 10.1007/s11033-023-08931-2. Mol Biol Rep. 2023. PMID: 38085375 Free PMC article. Review.
-
A Single Nucleotide Polymorphism in HPGD Gene Is Associated with Prostate Cancer Risk.J Cancer. 2017 Oct 24;8(19):4083-4086. doi: 10.7150/jca.22025. eCollection 2017. J Cancer. 2017. PMID: 29187884 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

