Intestinal organoids: a model of intestinal fibrosis for evaluating anti-fibrotic drugs
- PMID: 25828392
- PMCID: PMC5915372
- DOI: 10.1016/j.yexmp.2015.03.033
Intestinal organoids: a model of intestinal fibrosis for evaluating anti-fibrotic drugs
Abstract
Background & aims: Intestinal fibrosis is a critical complication of Crohn's disease (CD). Current in vitro models of intestinal fibrosis cannot model the complex intestinal architecture, while in vivo rodent models do not fully recapitulate human disease and have limited utility for large-scale screening. Here, we exploit recent advances in stem cell derived human intestinal organoids (HIOs) as a new human model of fibrosis in CD.
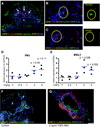
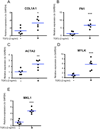
Methods: Human pluripotent stem cells were differentiated into HIOs. We identified myofibroblasts, the key effector cells of fibrosis, by immunofluorescence staining for alpha-smooth muscle actin (αSMA), vimentin, and desmin. We examined the fibrogenic response of HIOs by treatment with transforming growth factor beta (TGFβ) in the presence or absence of the anti-fibrotic drug spironolactone. Fibrotic response was assayed by expression of fibrogenic genes (COL1A1 (collagen, type I, alpha 1), ACTA2 (alpha smooth muscle actin), FN1 (fibronectin 1), MYLK (myosin light chain kinase), and MKL1 (megakaryoblastic leukemia (translocation) 1)) and proteins (αSMA).
Results: Immunofluorescent staining of organoids identified a population of myofibroblasts within the HIO mesenchyme. TGFβ stimulation of HIOs produced a dose-dependent pro-fibrotic response. Spironolactone treatment blocked the fibrogenic response of HIOs to TGFβ.
Conclusions: HIOs contain myofibroblasts and respond to a pro-fibrotic stimulus in a manner that is consistent with isolated human myofibroblasts. HIOs are a promising model system that might bridge the gap between current in vitro and in vivo models of intestinal fibrosis in IBD.
Keywords: Drug screening; Fibrosis model; Myofibroblasts; Organoid; TGFβ.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Similar articles
-
Umbilical cord/placenta-derived mesenchymal stem cells inhibit fibrogenic activation in human intestinal myofibroblasts via inhibition of myocardin-related transcription factor A.Stem Cell Res Ther. 2019 Sep 23;10(1):291. doi: 10.1186/s13287-019-1385-8. Stem Cell Res Ther. 2019. PMID: 31547873 Free PMC article.
-
Application of Human Induced Pluripotent Stem Cell-Derived Intestinal Organoids as a Model of Epithelial Damage and Fibrosis in Inflammatory Bowel Disease.Biol Pharm Bull. 2020;43(7):1088-1095. doi: 10.1248/bpb.b20-00088. Biol Pharm Bull. 2020. PMID: 32612071
-
Development of a Personalized Intestinal Fibrosis Model Using Human Intestinal Organoids Derived From Induced Pluripotent Stem Cells.Inflamm Bowel Dis. 2022 May 4;28(5):667-679. doi: 10.1093/ibd/izab292. Inflamm Bowel Dis. 2022. PMID: 34918082 Free PMC article.
-
Building additional complexity to in vitro-derived intestinal tissues.Stem Cell Res Ther. 2013;4 Suppl 1(Suppl 1):S1. doi: 10.1186/scrt362. Epub 2013 Dec 20. Stem Cell Res Ther. 2013. PMID: 24565179 Free PMC article. Review.
-
Engineered Synthetic Matrices for Human Intestinal Organoid Culture and Therapeutic Delivery.Adv Mater. 2024 Mar;36(9):e2307678. doi: 10.1002/adma.202307678. Epub 2023 Dec 6. Adv Mater. 2024. PMID: 37987171 Review.
Cited by
-
Organoid Models of Colorectal Pathology: Do They Hold the Key to Personalized Medicine? A Systematic Review.Dis Colon Rectum. 2020 Nov;63(11):1559-1569. doi: 10.1097/DCR.0000000000001806. Dis Colon Rectum. 2020. PMID: 32868555 Free PMC article.
-
The integrated transcriptome bioinformatics analysis identifies key genes and cellular components for proliferative diabetic retinopathy.PLoS One. 2022 Nov 21;17(11):e0277952. doi: 10.1371/journal.pone.0277952. eCollection 2022. PLoS One. 2022. PMID: 36409751 Free PMC article.
-
A human liver organoid screening platform for DILI risk prediction.J Hepatol. 2023 May;78(5):998-1006. doi: 10.1016/j.jhep.2023.01.019. Epub 2023 Feb 3. J Hepatol. 2023. PMID: 36738840 Free PMC article.
-
Intestinal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases.World J Gastroenterol. 2019 Aug 14;25(30):4125-4147. doi: 10.3748/wjg.v25.i30.4125. World J Gastroenterol. 2019. PMID: 31435168 Free PMC article. Review.
-
Engineering organoids.Nat Rev Mater. 2021;6(5):402-420. doi: 10.1038/s41578-021-00279-y. Epub 2021 Feb 19. Nat Rev Mater. 2021. PMID: 33623712 Free PMC article. Review.
References
-
- Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–281. vii. - PubMed
-
- Astashkina A, Grainger DW. Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev. 2014;69–70:1–18. - PubMed
-
- Chen X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

