Oral tolerance failure upon neonatal gut colonization with Escherichia coli producing the genotoxin colibactin
- PMID: 25824839
- PMCID: PMC4432747
- DOI: 10.1128/IAI.00064-15
Oral tolerance failure upon neonatal gut colonization with Escherichia coli producing the genotoxin colibactin
Abstract
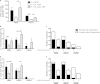
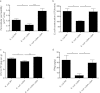
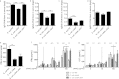
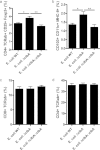
The intestinal barrier controls the balance between tolerance and immunity to luminal antigens. When this finely tuned equilibrium is deregulated, inflammatory disorders can occur. There is a concomitant increase, in urban populations of developed countries, of immune-mediated diseases along with a shift in Escherichia coli population from the declining phylogenetic group A to the newly dominant group B2, including commensal strains producing a genotoxin called colibactin that massively colonized the gut of neonates. Here, we showed that mother-to-offspring early gut colonization by colibactin-producing E. coli impairs intestinal permeability and enhances the transepithelial passage of luminal antigen, leading to an increased immune activation. Functionally, this was accompanied by a dramatic increase in local and systemic immune responses against a fed antigen, decreased regulatory T cell population, tolerogenic dendritic cells, and enhanced mucosal delayed-type hypersensitivity response. Conversely, the abolition of colibactin expression by mutagenesis abrogates the alteration of oral tolerance induced by neonatal colonization by E. coli. In conclusion, the vertical colonization by E. coli producing the genotoxin colibactin enhances intestinal translocation and subsequently alters oral tolerance. Thus, early colonization by E. coli from the newly dominant phylogenetic group B2, which produces colibactin, may represent a risk factor for the development of immune-mediated diseases.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
The Food Contaminant Deoxynivalenol Exacerbates the Genotoxicity of Gut Microbiota.mBio. 2017 Mar 14;8(2):e00007-17. doi: 10.1128/mBio.00007-17. mBio. 2017. PMID: 28292979 Free PMC article.
-
Maternally acquired genotoxic Escherichia coli alters offspring's intestinal homeostasis.Gut Microbes. 2014 May-Jun;5(3):313-25. doi: 10.4161/gmic.28932. Epub 2014 Jun 27. Gut Microbes. 2014. PMID: 24971581 Free PMC article.
-
The Genotoxin Colibactin Is a Determinant of Virulence in Escherichia coli K1 Experimental Neonatal Systemic Infection.Infect Immun. 2015 Sep;83(9):3704-11. doi: 10.1128/IAI.00716-15. Epub 2015 Jul 6. Infect Immun. 2015. PMID: 26150540 Free PMC article.
-
Early settlers: which E. coli strains do you not want at birth?Am J Physiol Gastrointest Liver Physiol. 2016 Jul 1;311(1):G123-9. doi: 10.1152/ajpgi.00091.2016. Epub 2016 Jun 10. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27288422 Review.
-
Interplay between siderophores and colibactin genotoxin in Escherichia coli.IUBMB Life. 2017 Jun;69(6):435-441. doi: 10.1002/iub.1612. Epub 2017 Mar 14. IUBMB Life. 2017. PMID: 28295919 Review.
Cited by
-
The abundance of Ruminococcus bromii is associated with faecal butyrate levels and atopic dermatitis in infancy.Allergy. 2022 Aug 2;77(12):3629-40. doi: 10.1111/all.15440. Online ahead of print. Allergy. 2022. PMID: 35917214 Free PMC article.
-
Bacterial Toxins Are a Never-Ending Source of Surprises: From Natural Born Killers to Negotiators.Toxins (Basel). 2021 Jun 17;13(6):426. doi: 10.3390/toxins13060426. Toxins (Basel). 2021. PMID: 34204481 Free PMC article. Review.
-
Colibactin: More Than a New Bacterial Toxin.Toxins (Basel). 2018 Apr 10;10(4):151. doi: 10.3390/toxins10040151. Toxins (Basel). 2018. PMID: 29642622 Free PMC article. Review.
-
The Role of Virulence Factors in Neonatal Sepsis Caused by Enterobacterales: A Systematic Review.Int J Mol Sci. 2022 Oct 8;23(19):11930. doi: 10.3390/ijms231911930. Int J Mol Sci. 2022. PMID: 36233238 Free PMC article. Review.
-
ClbG in Avian Pathogenic Escherichia coli Contributes to Meningitis Development in a Mouse Model.Toxins (Basel). 2021 Aug 6;13(8):546. doi: 10.3390/toxins13080546. Toxins (Basel). 2021. PMID: 34437417 Free PMC article.
References
-
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, et al. . 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65. doi:10.1038/nature08821. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

