MOG cell-based assay detects non-MS patients with inflammatory neurologic disease
- PMID: 25821844
- PMCID: PMC4370386
- DOI: 10.1212/NXI.0000000000000089
MOG cell-based assay detects non-MS patients with inflammatory neurologic disease
Abstract
Objective: To optimize sensitivity and disease specificity of a myelin oligodendrocyte glycoprotein (MOG) antibody assay.
Methods: Consecutive sera (n = 1,109) sent for aquaporin-4 (AQP4) antibody testing were screened for MOG antibodies (Abs) by cell-based assays using either full-length human MOG (FL-MOG) or the short-length form (SL-MOG). The Abs were initially detected by Alexa Fluor goat anti-human IgG (H + L) and subsequently by Alexa Fluor mouse antibodies to human IgG1.
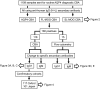
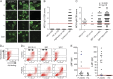
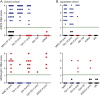
Results: When tested at 1:20 dilution, 40/1,109 sera were positive for AQP4-Abs, 21 for SL-MOG, and 180 for FL-MOG. Only one of the 40 AQP4-Ab-positive sera was positive for SL-MOG-Abs, but 10 (25%) were positive for FL-MOG-Abs (p = 0.0069). Of equal concern, 48% (42/88) of sera from controls (patients with epilepsy) were positive by FL-MOG assay. However, using an IgG1-specific secondary antibody, only 65/1,109 (5.8%) sera were positive on FL-MOG, and AQP4-Ab- positive and control sera were negative. IgM reactivity accounted for the remaining anti-human IgG (H + L) positivity toward FL-MOG. The clinical diagnoses were obtained in 33 FL-MOG-positive patients, blinded to the antibody data. IgG1-Abs to FL-MOG were associated with optic neuritis (n = 11), AQP4-seronegative neuromyelitis optica spectrum disorder (n = 4), and acute disseminated encephalomyelitis (n = 1). All 7 patients with probable multiple sclerosis (MS) were MOG-IgG1 negative.
Conclusions: The limited disease specificity of FL-MOG-Abs identified using Alexa Fluor goat anti-human IgG (H + L) is due in part to detection of IgM-Abs. Use of the FL-MOG and restricting to IgG1-Abs substantially improves specificity for non-MS demyelinating diseases.
Classification of evidence: This study provides Class II evidence that the presence of serum IgG1- MOG-Abs in AQP4-Ab-negative patients distinguishes non-MS CNS demyelinating disorders from MS (sensitivity 24%, 95% confidence interval [CI] 9%-45%; specificity 100%, 95% CI 88%-100%).
Figures

Comment in
-
Refining the Nosology of Antigen-Specific Diseases Within the Spectrum of Neuromyelitis Optica.Mult Scler Relat Disord. 2018 Oct;25:A1-A2. doi: 10.1016/j.msard.2018.09.027. Mult Scler Relat Disord. 2018. PMID: 30384960 No abstract available.
Similar articles
-
Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study.JAMA Neurol. 2014 Mar;71(3):276-83. doi: 10.1001/jamaneurol.2013.5857. JAMA Neurol. 2014. PMID: 24425068
-
Relevance of antibodies to myelin oligodendrocyte glycoprotein in CSF of seronegative cases.Neurology. 2019 Nov 12;93(20):e1867-e1872. doi: 10.1212/WNL.0000000000008479. Epub 2019 Oct 23. Neurology. 2019. PMID: 31645473
-
Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study.J Neurol. 2017 Dec;264(12):2420-2430. doi: 10.1007/s00415-017-8635-4. Epub 2017 Oct 23. J Neurol. 2017. PMID: 29063242 Free PMC article.
-
Myelin Oligodendrocyte Glycoprotein: Deciphering a Target in Inflammatory Demyelinating Diseases.Front Immunol. 2017 May 8;8:529. doi: 10.3389/fimmu.2017.00529. eCollection 2017. Front Immunol. 2017. PMID: 28533781 Free PMC article. Review.
-
Myelin oligodendrocyte glycoprotein antibodies in neurological disease.Nat Rev Neurol. 2019 Feb;15(2):89-102. doi: 10.1038/s41582-018-0112-x. Nat Rev Neurol. 2019. PMID: 30559466 Review.
Cited by
-
The neuro-ophthalmological manifestations of NMOSD and MOGAD-a comprehensive review.Eye (Lond). 2023 Aug;37(12):2391-2398. doi: 10.1038/s41433-023-02477-0. Epub 2023 Mar 16. Eye (Lond). 2023. PMID: 36928226 Free PMC article. Review.
-
Outcome prediction models in AQP4-IgG positive neuromyelitis optica spectrum disorders.Brain. 2019 May 1;142(5):1310-1323. doi: 10.1093/brain/awz054. Brain. 2019. PMID: 30938427 Free PMC article.
-
Effects of the Positive Threshold and Data Analysis on Human MOG Antibody Detection by Live Flow Cytometry.Front Immunol. 2020 Feb 6;11:119. doi: 10.3389/fimmu.2020.00119. eCollection 2020. Front Immunol. 2020. PMID: 32117270 Free PMC article.
-
Seropositive anti-MOG antibody-associated acute disseminated encephalomyelitis (ADEM): a sequelae of Mycoplasma pneumoniae infection.BMJ Case Rep. 2020 May 19;13(5):e234565. doi: 10.1136/bcr-2020-234565. BMJ Case Rep. 2020. PMID: 32434879 Free PMC article.
-
Isolated new onset 'atypical' optic neuritis in the NMO clinic: serum antibodies, prognoses and diagnoses at follow-up.J Neurol. 2016 Feb;263(2):370-379. doi: 10.1007/s00415-015-7983-1. Epub 2015 Dec 14. J Neurol. 2016. PMID: 26668077
References
-
- Berger T, Rubner P, Schautzer F, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med 2003;349:139–145. - PubMed
-
- Zadro I, Brinar V, Horvat G, Brinar M. Clinical relevance of antibodies against myelin oligodendrocyte glycoprotein in different clinical types of multiple sclerosis. Clin Neurol Neurosurg 2007;109:23–26. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
