Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice
- PMID: 25818172
- PMCID: PMC4389244
- DOI: 10.1038/ncomms7623
Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice
Abstract
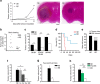
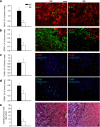
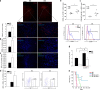
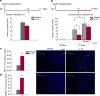
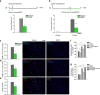
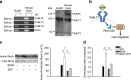
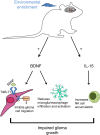
Mice exposed to standard (SE) or enriched environment (EE) were transplanted with murine or human glioma cells and differences in tumour development were evaluated. We report that EE exposure affects: (i) tumour size, increasing mice survival; (ii) glioma establishment, proliferation and invasion; (iii) microglia/macrophage (M/Mφ) activation; (iv) natural killer (NK) cell infiltration and activation; and (v) cerebral levels of IL-15 and BDNF. Direct infusion of IL-15 or BDNF in the brain of mice transplanted with glioma significantly reduces tumour growth. We demonstrate that brain infusion of IL-15 increases the frequency of NK cell infiltrating the tumour and that NK cell depletion reduces the efficacy of EE and IL-15 on tumour size and of EE on mice survival. BDNF infusion reduces M/Mφ infiltration and CD68 immunoreactivity in tumour mass and reduces glioma migration inhibiting the small G protein RhoA through the truncated TrkB.T1 receptor. These results suggest alternative approaches for glioma treatment.
Figures
Similar articles
-
Enriched Environment Cues Suggest a New Strategy to Counteract Glioma: Engineered rAAV2-IL-15 Microglia Modulate the Tumor Microenvironment.Front Immunol. 2021 Sep 6;12:730128. doi: 10.3389/fimmu.2021.730128. eCollection 2021. Front Immunol. 2021. PMID: 34552593 Free PMC article.
-
Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response.J Pathol. 2013 Jul;230(3):310-21. doi: 10.1002/path.4192. J Pathol. 2013. PMID: 23520016
-
The Glycoside Oleandrin Reduces Glioma Growth with Direct and Indirect Effects on Tumor Cells.J Neurosci. 2017 Apr 5;37(14):3926-3939. doi: 10.1523/JNEUROSCI.2296-16.2017. Epub 2017 Mar 14. J Neurosci. 2017. PMID: 28292827 Free PMC article.
-
The role of microglia and macrophages in glioma maintenance and progression.Nat Neurosci. 2016 Jan;19(1):20-7. doi: 10.1038/nn.4185. Nat Neurosci. 2016. PMID: 26713745 Free PMC article. Review.
-
Enhancing Effects of Environmental Enrichment on the Functions of Natural Killer Cells in Mice.Front Immunol. 2021 Jul 28;12:695859. doi: 10.3389/fimmu.2021.695859. eCollection 2021. Front Immunol. 2021. PMID: 34394087 Free PMC article. Review.
Cited by
-
KCa3.1 inhibition switches the phenotype of glioma-infiltrating microglia/macrophages.Cell Death Dis. 2016 Apr 7;7(4):e2174. doi: 10.1038/cddis.2016.73. Cell Death Dis. 2016. PMID: 27054329 Free PMC article.
-
Elevated Na/H exchanger 1 (SLC9A1) emerges as a marker for tumorigenesis and prognosis in gliomas.J Exp Clin Cancer Res. 2018 Oct 17;37(1):255. doi: 10.1186/s13046-018-0923-z. J Exp Clin Cancer Res. 2018. PMID: 30333031 Free PMC article.
-
Targeting the BDNF/TrkB pathway for the treatment of tumors.Oncol Lett. 2019 Feb;17(2):2031-2039. doi: 10.3892/ol.2018.9854. Epub 2018 Dec 20. Oncol Lett. 2019. PMID: 30675270 Free PMC article. Review.
-
Enriched Environment Cues Suggest a New Strategy to Counteract Glioma: Engineered rAAV2-IL-15 Microglia Modulate the Tumor Microenvironment.Front Immunol. 2021 Sep 6;12:730128. doi: 10.3389/fimmu.2021.730128. eCollection 2021. Front Immunol. 2021. PMID: 34552593 Free PMC article.
-
All Wrapped Up: Environmental Effects on Myelination.Trends Neurosci. 2017 Sep;40(9):572-587. doi: 10.1016/j.tins.2017.06.009. Epub 2017 Aug 22. Trends Neurosci. 2017. PMID: 28844283 Free PMC article. Review.
References
-
- Nithianantharajah J. & Hannan A. J. The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Prog. Neurobiol. 89, 369–382 (2009) . - PubMed
-
- Sale A., Berardi N. & Maffei L. Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiol. Rev. 94, 189–234 (2014) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

