Switching roles: the functional plasticity of adult tissue stem cells
- PMID: 25812989
- PMCID: PMC4426478
- DOI: 10.15252/embj.201490386
Switching roles: the functional plasticity of adult tissue stem cells
Abstract
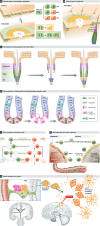
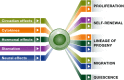
Adult organisms have to adapt to survive, and the same is true for their tissues. Rates and types of cell production must be rapidly and reversibly adjusted to meet tissue demands in response to both local and systemic challenges. Recent work reveals how stem cell (SC) populations meet these requirements by switching between functional states tuned to homoeostasis or regeneration. This plasticity extends to differentiating cells, which are capable of reverting to SCs after injury. The concept of the niche, the micro-environment that sustains and regulates stem cells, is broadening, with a new appreciation of the role of physical factors and hormonal signals. Here, we review different functions of SCs, the cellular mechanisms that underlie them and the signals that bias the fate of SCs as they switch between roles.
Keywords: differentiation; niche; regeneration; signal transduction; stem cells.
© 2015 The Authors.
Figures

Similar articles
-
Erratum: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs.J Vis Exp. 2023 May 26;(195). doi: 10.3791/6561. J Vis Exp. 2023. PMID: 37235796
-
Peer Play.2023 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30020595 Free Books & Documents.
-
Ethics of Procuring and Using Organs or Tissue from Infants and Newborns for Transplantation, Research, or Commercial Purposes: Protocol for a Bioethics Scoping Review.Wellcome Open Res. 2024 Dec 5;9:717. doi: 10.12688/wellcomeopenres.23235.1. eCollection 2024. Wellcome Open Res. 2024. PMID: 39839977 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Vascular Wall as Source of Stem Cells Able to Differentiate into Endothelial Cells.Adv Exp Med Biol. 2020;1237:29-36. doi: 10.1007/5584_2019_421. Adv Exp Med Biol. 2020. PMID: 31797283 Review.
-
Behavior and biocompatibility of rabbit bone marrow mesenchymal stem cells with bacterial cellulose membrane.PeerJ. 2018 Apr 30;6:e4656. doi: 10.7717/peerj.4656. eCollection 2018. PeerJ. 2018. PMID: 29736332 Free PMC article.
-
First immunohistochemical evidence of human tendon repair following stem cell injection: A case report and review of literature.World J Stem Cells. 2021 Jul 26;13(7):944-970. doi: 10.4252/wjsc.v13.i7.944. World J Stem Cells. 2021. PMID: 34367486 Free PMC article.
-
EMT Involved in Migration of Stem/Progenitor Cells for Pituitary Development and Regeneration.J Clin Med. 2016 Apr 6;5(4):43. doi: 10.3390/jcm5040043. J Clin Med. 2016. PMID: 27058562 Free PMC article. Review.
-
Artificial gametes from stem cells.Clin Exp Reprod Med. 2015 Jun;42(2):33-44. doi: 10.5653/cerm.2015.42.2.33. Epub 2015 Jun 30. Clin Exp Reprod Med. 2015. PMID: 26161331 Free PMC article. Review.
References
-
- Alcolea MP, Jones PH. Tracking cells in their native habitat: lineage tracing in epithelial neoplasia. Nat Rev Cancer. 2013;13:161–171. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

