Comparison of epigenetic versus standard induction chemotherapy for newly diagnosed acute myeloid leukemia patients ≥60 years old
- PMID: 25808347
- PMCID: PMC6791130
- DOI: 10.1002/ajh.24016
Comparison of epigenetic versus standard induction chemotherapy for newly diagnosed acute myeloid leukemia patients ≥60 years old
Abstract
Older patients with acute myeloid leukemia (AML) have poor outcomes with standard induction chemotherapy. We retrospectively reviewed our institute's experience with epigenetic (Epi) versus cytarabine- and anthracycline-based intensive chemotherapy (IC) as induction in newly diagnosed AML patients aged 60 years and older. One hundred sixty-seven patients (n = 84, IC; n = 83, Epi) were assessed; 69 patients received decitabine and 14 azacitidine. Baseline characteristics between the IC and Epi patient cohorts were not statistically different except for age, initial white blood cell count, and comorbidity index. Overall response rate (ORR, 50% vs. 28%, respectively, P < 0.01) and complete response rate (CRR, 43% vs. 20%, respectively, P < 0.01) were superior following IC vs. Epi. Although univariate analysis demonstrated longer overall survival after IC (10.7 vs. 9.1 months, P = 0.012), multivariate analysis showed no independent impact of induction treatment. Treatment-related mortality was not statistically different in the two groups. Outcomes of patients with secondary, poor cytogenetic risk, FLT-3 mutated AML, or relapsed/refractory disease after IC or Epi were not significantly different. Outcomes of patients receiving IC versus a 10-day decitabine regimen (n = 63) also were not significantly different. Our results suggest that IC and Epi therapy are clinically equivalent approaches for upfront treatment of older patients with AML and that other factors (feasibility, toxicity, cost, etc.) should drive treatment decisions. Prospective randomized trials to determine the optimal induction approach for specific patient subsets are needed.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest
The authors report no potential conflicts of interest.
Figures
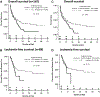
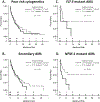
Similar articles
-
Characteristics and outcomes of older patients with secondary acute myeloid leukemia according to treatment approach.Cancer. 2017 Aug 15;123(16):3050-3060. doi: 10.1002/cncr.30704. Epub 2017 Apr 7. Cancer. 2017. PMID: 28387922 Free PMC article.
-
Intensive chemotherapy vs. hypomethylating agents in older adults with newly diagnosed high-risk acute myeloid leukemia: A single center experience.Leuk Res. 2018 Dec;75:29-35. doi: 10.1016/j.leukres.2018.10.011. Epub 2018 Oct 25. Leuk Res. 2018. PMID: 30445237 Free PMC article.
-
Decitabine Versus Intensive Chemotherapy for Elderly Patients With Newly Diagnosed Acute Myeloid Leukemia.Clin Lymphoma Myeloma Leuk. 2019 May;19(5):290-299.e3. doi: 10.1016/j.clml.2019.02.002. Epub 2019 Feb 20. Clin Lymphoma Myeloma Leuk. 2019. PMID: 30879987
-
The euphoria of hypomethylating agents in MDS and AML: is it justified?Best Pract Res Clin Haematol. 2013 Sep;26(3):275-8. doi: 10.1016/j.beha.2013.10.001. Epub 2013 Oct 15. Best Pract Res Clin Haematol. 2013. PMID: 24309530 Review.
-
Clinical status of induction therapy incorporating a hypomethylating agent for newly diagnosed adult acute myeloid leukemia compared to the standard 7+3 regimen.Expert Rev Hematol. 2023 Jul-Dec;16(10):761-771. doi: 10.1080/17474086.2023.2256472. Epub 2023 Sep 8. Expert Rev Hematol. 2023. PMID: 37670667 Review.
Cited by
-
The prognostic significance of genetics in acute myeloid leukemia under venetoclax-based treatment.Ann Hematol. 2024 Dec;103(12):5019-5033. doi: 10.1007/s00277-024-06050-x. Epub 2024 Oct 29. Ann Hematol. 2024. PMID: 39467855 Review.
-
Bridging Strategies to Allogeneic Transplant for Older AML Patients.Cancers (Basel). 2018 Jul 11;10(7):232. doi: 10.3390/cancers10070232. Cancers (Basel). 2018. PMID: 29997333 Free PMC article. Review.
-
Induction of cancer testis antigen expression in circulating acute myeloid leukemia blasts following hypomethylating agent monotherapy.Oncotarget. 2016 Mar 15;7(11):12840-56. doi: 10.18632/oncotarget.7326. Oncotarget. 2016. PMID: 26883197 Free PMC article.
-
Intensity of chemotherapy for the initial management of newly diagnosed acute myeloid leukemia in older patients.Future Oncol. 2019 Jun;15(17):1989-1995. doi: 10.2217/fon-2019-0001. Epub 2019 Jun 7. Future Oncol. 2019. PMID: 31170814 Free PMC article.
-
Clinical Outcomes of 217 Patients with Acute Erythroleukemia According to Treatment Type and Line: A Retrospective Multinational Study.Int J Mol Sci. 2017 Apr 14;18(4):837. doi: 10.3390/ijms18040837. Int J Mol Sci. 2017. PMID: 28420120 Free PMC article.
References
-
- Lofgren C, Albertioni F, Paul C. High activity and incomplete cross resistance of nucleoside analogues cladribine and fludarabine versus Ara-C on leukemic cells from patients with AML. Ther Drug Monit 2005;27:641–646. - PubMed
-
- Estey E, Döhner H. Acute myeloid leukaemia. Lancet 2006;368:1894–1907. - PubMed
-
- Latagliata R, Petti MC, Mandelli F. Acute myeloid leukemia in the elderly: ‘Per aspera ad astra’? Leuk Res 1999;23:603–613. - PubMed
-
- Sekeres MA. Treatment of older adults with acute myeloid leukemia: State of the art and current perspectives. Haematologica 2008;93:1769–1772. - PubMed
-
- Leith CP, Kopecky KJ, Chen IM, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: A Southwest Oncology Group Study. Blood 1999;94:1086–1099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
