Intracellular detection of viral nucleic acids
- PMID: 25795286
- PMCID: PMC5084527
- DOI: 10.1016/j.mib.2015.03.001
Intracellular detection of viral nucleic acids
Abstract
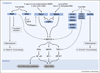
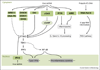
Successful clearance of a microbial infection depends on the concerted action of both the innate and adaptive arms of the immune system. Accurate recognition of an invading pathogen is the first and most crucial step in eliciting effective antimicrobial defense mechanisms. In recent years, remarkable progress has been made towards understanding the molecular details of how the innate immune system recognizes microbial signatures, commonly called pathogen-associated molecular patterns (PAMPs). For viral pathogens, nucleic acids-both viral genomes and viral replication products-represent a major class of PAMPs that trigger antiviral host responses via activation of germline-encoded innate immune receptors. Here we summarize recent advances in intracellular innate sensing mechanisms of viral RNA and DNA.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Identification of a Natural Viral RNA Motif That Optimizes Sensing of Viral RNA by RIG-I.mBio. 2015 Oct 6;6(5):e01265-15. doi: 10.1128/mBio.01265-15. mBio. 2015. PMID: 26443454 Free PMC article.
-
Emerging complexity and new roles for the RIG-I-like receptors in innate antiviral immunity.Virol Sin. 2015 Jun;30(3):163-73. doi: 10.1007/s12250-015-3604-5. Epub 2015 May 20. Virol Sin. 2015. PMID: 25997992 Free PMC article. Review.
-
Pattern recognition of viral nucleic acids by RIG-I-like helicases.J Mol Med (Berl). 2011 Jan;89(1):5-12. doi: 10.1007/s00109-010-0672-8. Epub 2010 Sep 4. J Mol Med (Berl). 2011. PMID: 20820752 Review.
-
The role of viral nucleic acid recognition in dendritic cells for innate and adaptive antiviral immunity.Immunobiology. 2007;212(9-10):701-14. doi: 10.1016/j.imbio.2007.09.007. Epub 2007 Nov 9. Immunobiology. 2007. PMID: 18086372 Free PMC article. Review.
-
Innate recognition of viruses.Immunol Lett. 2010 Jan 18;128(1):17-20. doi: 10.1016/j.imlet.2009.09.010. Epub 2009 Oct 4. Immunol Lett. 2010. PMID: 19808054 Review.
Cited by
-
Structural aspects of nucleic acid-sensing Toll-like receptors.Biophys Rev. 2016 Mar;8(1):33-43. doi: 10.1007/s12551-015-0187-1. Epub 2016 Feb 12. Biophys Rev. 2016. PMID: 28510149 Free PMC article. Review.
-
Structure and Function of Viral Deubiquitinating Enzymes.J Mol Biol. 2017 Nov 10;429(22):3441-3470. doi: 10.1016/j.jmb.2017.06.010. Epub 2017 Jun 16. J Mol Biol. 2017. PMID: 28625850 Free PMC article. Review.
-
Outrunning the Red Queen: bystander activation as a means of outpacing innate immune subversion by intracellular pathogens.Cell Mol Immunol. 2017 Jan;14(1):14-21. doi: 10.1038/cmi.2016.36. Epub 2016 Aug 22. Cell Mol Immunol. 2017. PMID: 27545071 Free PMC article. Review.
-
Actin cytoskeleton remodeling primes RIG-I-like receptor activation.Cell. 2022 Sep 15;185(19):3588-3602.e21. doi: 10.1016/j.cell.2022.08.011. Cell. 2022. PMID: 36113429 Free PMC article.
-
Interferon antagonists encoded by SARS-CoV-2 at a glance.Med Microbiol Immunol. 2023 Apr;212(2):125-131. doi: 10.1007/s00430-022-00734-9. Epub 2022 Apr 2. Med Microbiol Immunol. 2023. PMID: 35366686 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

