COMP-Ang1 Potentiates EPC Treatment of Ischemic Brain Injury by Enhancing Angiogenesis Through Activating AKT-mTOR Pathway and Promoting Vascular Migration Through Activating Tie2-FAK Pathway
- PMID: 25792870
- PMCID: PMC4363334
- DOI: 10.5607/en.2015.24.1.55
COMP-Ang1 Potentiates EPC Treatment of Ischemic Brain Injury by Enhancing Angiogenesis Through Activating AKT-mTOR Pathway and Promoting Vascular Migration Through Activating Tie2-FAK Pathway
Abstract
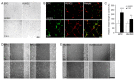
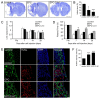
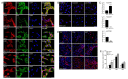
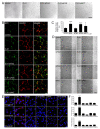
Successful recovery from brain ischemia is limited due to poor vascularization surrounding the ischemic zone. Cell therapy with strong angiogenic factors could be an effective strategy to rescue the ischemic brain. We investigated whether cartilage oligomeric matrix protein (COMP)-Ang1, a soluble, stable and potent Ang1 variant, enhances the angiogenesis of human cord blood derived endothelial progenitor cells (hCB-EPCs) for rescuing brain from ischemic injury. COMP-Ang1 markedly improved the tube formation of capillaries by EPCs and incorporation of EPCs into tube formation with human umbilical vein endothelial cells (HUVECs) upon incubation on matrigel in vitro. COMP-Ang1 stimulated the migration of EPCs more than HUVECs in a scratch wound migration assay. The transplanted EPCs and COMP-Ang1 were incorporated into the blood vessels and decreased the infarct volume in the rat ischemic brain. Molecular studies revealed that COMP-Ang1 induced an interaction between Tie2 and FAK, but AKT was separated from the Tie2-FAK-AKT complex in the EPC plasma membrane. Tie2-FAK increased pp38, pSAPK/JNK, and pERK-mediated MAPK activation and interacted with integrins ανβ3, α4, β1, finally leading to migration of EPCs. AKT recruited mTOR, SDF-1, and HIF-1α to induce angiogenesis. Taken together, it is concluded that COMP-Ang1 potentiates the angiogenesis of EPCs and enhances the vascular morphogenesis indicating that combination of EPCs with COMP-Ang1 may be a potentially effective regimen for ischemic brain injury salvage therapy.
Keywords: COMP-Ang1; Tie2-FAK-AKT pathway; angiogenesis; ischemia.
Figures
Similar articles
-
COMP-Ang1 stimulates HIF-1α-mediated SDF-1 overexpression and recovers ischemic injury through BM-derived progenitor cell recruitment.Blood. 2011 Apr 21;117(16):4376-86. doi: 10.1182/blood-2010-07-295964. Epub 2011 Jan 3. Blood. 2011. PMID: 21200018
-
Protective role of COMP-Ang1 in ischemic rat brain.J Neurosci Res. 2010 Apr;88(5):1052-63. doi: 10.1002/jnr.22274. J Neurosci Res. 2010. PMID: 19885826
-
COMP-Ang1 enhances DNA synthesis and cell cycle progression in human periodontal ligament cells via Tie2-mediated phosphorylation of PI3K/Akt and MAPKs.Mol Cell Biochem. 2016 May;416(1-2):157-68. doi: 10.1007/s11010-016-2704-3. Epub 2016 Apr 23. Mol Cell Biochem. 2016. PMID: 27107990
-
COMP-Ang1 inhibits apoptosis as well as improves the attenuated osteogenic differentiation of mesenchymal stem cells induced by advanced glycation end products.Biochim Biophys Acta. 2013 Oct;1830(10):4928-34. doi: 10.1016/j.bbagen.2013.06.035. Epub 2013 Jul 10. Biochim Biophys Acta. 2013. PMID: 23850469
-
COMP-Ang1: Therapeutic potential of an engineered Angiopoietin-1 variant.Vascul Pharmacol. 2021 Dec;141:106919. doi: 10.1016/j.vph.2021.106919. Epub 2021 Sep 25. Vascul Pharmacol. 2021. PMID: 34583025 Review.
Cited by
-
Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR.Neural Regen Res. 2016 Mar;11(3):372-85. doi: 10.4103/1673-5374.179032. Neural Regen Res. 2016. PMID: 27127460 Free PMC article. Review.
-
Pivotal micro factors associated with endothelial cells.Chin Med J (Engl). 2019 Aug 20;132(16):1965-1973. doi: 10.1097/CM9.0000000000000358. Chin Med J (Engl). 2019. PMID: 31335473 Free PMC article. Review.
-
Intravitreal AAV2.COMP-Ang1 Prevents Neurovascular Degeneration in a Murine Model of Diabetic Retinopathy.Diabetes. 2015 Dec;64(12):4247-59. doi: 10.2337/db14-1030. Epub 2015 Sep 4. Diabetes. 2015. PMID: 26340930 Free PMC article.
-
Regeneration in the nervous system with erythropoietin.Front Biosci (Landmark Ed). 2016 Jan;21(3):561-596. doi: 10.2741/4408. Front Biosci (Landmark Ed). 2016. PMID: 26549969 Free PMC article.
-
WISP-1 positively regulates angiogenesis by controlling VEGF-A expression in human osteosarcoma.Cell Death Dis. 2017 Apr 13;8(4):e2750. doi: 10.1038/cddis.2016.421. Cell Death Dis. 2017. PMID: 28406476 Free PMC article.
References
-
- Yanagisawa-Miwa A, Uchida Y, Nakamura F, Tomaru T, Kido H, Kamijo T, Sugimoto T, Kaji K, Utsuyama M, Kurashima C, Ito H. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science. 1992;257:1401–1403. - PubMed
-
- Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. - PubMed
-
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. - PubMed
-
- Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. - PubMed
-
- Cho CH, Kim KE, Byun J, Jang HS, Kim DK, Baluk P, Baffert F, Lee GM, Mochizuki N, Kim J, Jeon BH, McDonald DM, Koh GY. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ Res. 2005;97:86–94. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

