Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway
- PMID: 25792811
- PMCID: PMC4362906
- DOI: 10.2147/DDDT.S74197
Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway
Abstract
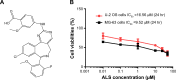
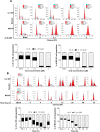
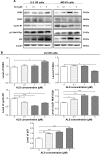
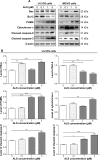
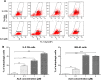
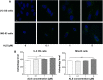
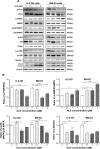
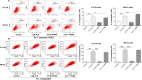
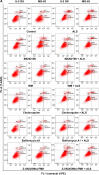
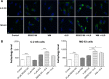
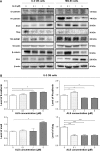
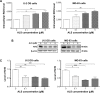
Osteosarcoma (OS) is the most common malignant bone tumor occurring mostly in children and adolescents between 10 and 20 years of age with poor response to current therapeutics. Alisertib (ALS, MLN8237) is a selective Aurora kinase A inhibitor that displays anticancer effects on several types of cancer. However, the role of ALS in the treatment of OS remains unknown. This study aimed to investigate the effects of ALS on the cell growth, apoptosis, autophagy, and epithelial to mesenchymal transition (EMT) and the underlying mechanisms in two human OS cell lines U-2 OS and MG-63. The results showed that ALS had potent growth inhibitory, pro-apoptotic, pro-autophagic, and EMT inhibitory effects on U-2 OS and MG-63 cells. ALS remarkably induced G2/M arrest and down-regulated the expression levels of cyclin-dependent kinases 1 and 2 and cyclin B1 in both U-2 OS and MG-63 cells. ALS markedly induced mitochondria-mediated apoptosis with a significant increase in the expression of key pro-apoptotic proteins and a decrease in main anti-apoptotic proteins. Furthermore, ALS promoted autophagic cell death via the inhibition of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) and p38 mitogen-activated protein kinase (p38 MAPK) signaling pathways, and activation of 5'-AMP-dependent kinase (AMPK) signaling pathway. Inducers or inhibitors of apoptosis or autophagy simultaneously altered ALS-induced apoptotic and autophagic death in both U-2 OS and MG-63 cells, suggesting a crosstalk between these two primary modes of programmed cell death. Moreover, ALS suppressed EMT-like phenotypes with a marked increase in the expression of E-cadherin but a decrease in N-cadherin in U-2 OS and MG-63 cells. ALS treatment also induced reactive oxygen species (ROS) generation but inhibited the expression levels of sirtuin 1 and nuclear factor-erythroid-2-related factor 2 (Nrf2) in both cell lines. Taken together, these findings show that ALS promotes apoptosis and autophagy but inhibits EMT via PI3K/Akt/mTOR, p38 MAPK, and AMPK signaling pathways with involvement of ROS- and sirtuin 1-associated pathways in U-2 OS and MG-63 cells. ALS is a promising anticancer agent in OS treatment and further studies are needed to confirm its efficacy and safety in OS chemotherapy.
Keywords: ALS; EMT; PI3K/Akt/mTOR pathway; apoptosis; autophagy; osteosarcoma.
Figures








Similar articles
-
The investigational Aurora kinase A inhibitor alisertib (MLN8237) induces cell cycle G2/M arrest, apoptosis, and autophagy via p38 MAPK and Akt/mTOR signaling pathways in human breast cancer cells.Drug Des Devel Ther. 2015 Mar 16;9:1627-52. doi: 10.2147/DDDT.S75378. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25834401 Free PMC article.
-
Alisertib induces cell cycle arrest and autophagy and suppresses epithelial-to-mesenchymal transition involving PI3K/Akt/mTOR and sirtuin 1-mediated signaling pathways in human pancreatic cancer cells.Drug Des Devel Ther. 2015 Jan 17;9:575-601. doi: 10.2147/DDDT.S75221. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25632225 Free PMC article.
-
Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells.Drug Des Devel Ther. 2015 Jan 9;9:425-64. doi: 10.2147/DDDT.S74062. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25624750 Free PMC article.
-
PI3K/Akt/mTOR pathway inhibitors in cancer: a perspective on clinical progress.Curr Med Chem. 2010;17(35):4326-41. doi: 10.2174/092986710793361234. Curr Med Chem. 2010. PMID: 20939811 Review.
-
The PI3K/AKT/mTOR signaling pathway inhibitors enhance radiosensitivity in cancer cell lines.Mol Biol Rep. 2021 Aug;48(8):1-14. doi: 10.1007/s11033-021-06607-3. Epub 2021 Aug 6. Mol Biol Rep. 2021. PMID: 34357550 Review.
Cited by
-
The Aurora kinase inhibitors in cancer research and therapy.J Cancer Res Clin Oncol. 2016 Sep;142(9):1995-2012. doi: 10.1007/s00432-016-2136-1. Epub 2016 Mar 1. J Cancer Res Clin Oncol. 2016. PMID: 26932147 Review.
-
Current Status and Prospects of Targeted Therapy for Osteosarcoma.Cells. 2022 Nov 5;11(21):3507. doi: 10.3390/cells11213507. Cells. 2022. PMID: 36359903 Free PMC article. Review.
-
Aurora kinase A revives dormant laryngeal squamous cell carcinoma cells via FAK/PI3K/Akt pathway activation.Oncotarget. 2016 Jul 26;7(30):48346-48359. doi: 10.18632/oncotarget.10233. Oncotarget. 2016. PMID: 27356739 Free PMC article.
-
EMMPRIN, SP1 and microRNA-27a mediate physcion 8-O-β-glucopyranoside-induced apoptosis in osteosarcoma cells.Am J Cancer Res. 2016 Jun 1;6(6):1331-44. eCollection 2016. Am J Cancer Res. 2016. Retraction in: Am J Cancer Res. 2020 Jul 01;10(7):2202. PMID: 27429847 Free PMC article. Retracted.
-
Analyzing the disease module associated with osteosarcoma via a network- and pathway-based approach.Exp Ther Med. 2018 Sep;16(3):2584-2592. doi: 10.3892/etm.2018.6506. Epub 2018 Jul 23. Exp Ther Med. 2018. PMID: 30210606 Free PMC article.
References
-
- Marulanda GA, Henderson ER, Johnson DA, Letson GD, Cheong D. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13–20. - PubMed
-
- Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–5. - PubMed
-
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. In: Jaffe, Bruland OS, Bielack S, editors. Pediatric and Adolescent Osteosarcoma. Springer; 2010. pp. 3–13.
-
- Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28(1–2):247–263. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

