UV Light Potentiates STING (Stimulator of Interferon Genes)-dependent Innate Immune Signaling through Deregulation of ULK1 (Unc51-like Kinase 1)
- PMID: 25792739
- PMCID: PMC4424351
- DOI: 10.1074/jbc.M115.649301
UV Light Potentiates STING (Stimulator of Interferon Genes)-dependent Innate Immune Signaling through Deregulation of ULK1 (Unc51-like Kinase 1)
Abstract
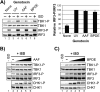
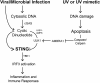
The mechanism by which ultraviolet (UV) wavelengths of sunlight trigger or exacerbate the symptoms of the autoimmune disorder lupus erythematosus is not known but may involve a role for the innate immune system. Here we show that UV radiation potentiates STING (stimulator of interferon genes)-dependent activation of the immune signaling transcription factor interferon regulatory factor 3 (IRF3) in response to cytosolic DNA and cyclic dinucleotides in keratinocytes and other human cells. Furthermore, we find that modulation of this innate immune response also occurs with UV-mimetic chemical carcinogens and in a manner that is independent of DNA repair and several DNA damage and cell stress response signaling pathways. Rather, we find that the stimulation of STING-dependent IRF3 activation by UV is due to apoptotic signaling-dependent disruption of ULK1 (Unc51-like kinase 1), a pro-autophagic protein that negatively regulates STING. Thus, deregulation of ULK1 signaling by UV-induced DNA damage may contribute to the negative effects of sunlight UV exposure in patients with autoimmune disorders.
Keywords: Apoptosis; Autoimmunity; Autophagy; Cell Signaling; DNA Damage; DNA Damage Response; DNA Repair; Innate Immunity; Interferon; Nucleotide Excision Repair.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







Similar articles
-
Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling.Cell. 2013 Oct 24;155(3):688-98. doi: 10.1016/j.cell.2013.09.049. Epub 2013 Oct 10. Cell. 2013. PMID: 24119841 Free PMC article.
-
DNA damage-triggered activation of cGAS-STING pathway induces apoptosis in human keratinocyte HaCaT cells.Mol Immunol. 2021 Mar;131:180-190. doi: 10.1016/j.molimm.2020.12.037. Epub 2021 Jan 8. Mol Immunol. 2021. PMID: 33423764
-
PELI2 is a negative regulator of STING signaling that is dynamically repressed during viral infection.Mol Cell. 2024 Jul 11;84(13):2423-2435.e5. doi: 10.1016/j.molcel.2024.06.001. Epub 2024 Jun 24. Mol Cell. 2024. PMID: 38917796
-
Crosstalk Between Apoptosis and Autophagy: Environmental Genotoxins, Infection, and Innate Immunity.J Cell Death. 2017 Feb 20;9:1179670716685085. doi: 10.1177/1179670716685085. eCollection 2017. J Cell Death. 2017. PMID: 28469477 Free PMC article. Review.
-
[Innate immune DNA sensing pathways].Uirusu. 2014;64(1):83-94. doi: 10.2222/jsv.64.83. Uirusu. 2014. PMID: 25765984 Review. Japanese.
Cited by
-
Human and Murine Evidence for Mechanisms Driving Autoimmune Photosensitivity.Front Immunol. 2018 Oct 23;9:2430. doi: 10.3389/fimmu.2018.02430. eCollection 2018. Front Immunol. 2018. PMID: 30405625 Free PMC article. Review.
-
STING protects against cardiac dysfunction and remodelling by blocking autophagy.Cell Commun Signal. 2021 Nov 8;19(1):109. doi: 10.1186/s12964-021-00793-0. Cell Commun Signal. 2021. PMID: 34749750 Free PMC article.
-
HERC6 regulates STING activity in a sex-biased manner through modulation of LATS2/VGLL3 Hippo signaling.iScience. 2024 Jan 23;27(2):108986. doi: 10.1016/j.isci.2024.108986. eCollection 2024 Feb 16. iScience. 2024. PMID: 38327798 Free PMC article.
-
Local Adaptation of Sun-Exposure-Dependent Gene Expression Regulation in Human Skin.PLoS Genet. 2016 Oct 19;12(10):e1006382. doi: 10.1371/journal.pgen.1006382. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27760139 Free PMC article.
-
Programmed Cell Death Pathways in the Pathogenesis of Systemic Lupus Erythematosus.J Immunol Res. 2019 Dec 1;2019:3638562. doi: 10.1155/2019/3638562. eCollection 2019. J Immunol Res. 2019. PMID: 31871956 Free PMC article. Review.
References
-
- Baer R. L., Harber L. C. (1965) Photobiology of lupus erythematosus. Arch. Dermatol. 92, 124–128 - PubMed
-
- Epstein J. H., Tuffanelli D., Dubois E. L. (1965) Light sensitivity and lupus erythematosus. Arch. Dermatol. 91, 483–485 - PubMed
-
- Barbhaiya M., Costenbader K. H. (2014) Ultraviolet radiation and systemic lupus erythematosus. Lupus 23, 588–595 - PubMed
-
- O'Neill L. A. (2013) Immunology. Sensing the dark side of DNA. Science 339, 763–764 - PubMed
-
- Cai X., Chiu Y. H., Chen Z. J. (2014) The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol. Cell 54, 289–296 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

