Calcium-sensing receptor stimulates Cl(-)- and SCFA-dependent but inhibits cAMP-dependent HCO3(-) secretion in colon
- PMID: 25792563
- PMCID: PMC4437021
- DOI: 10.1152/ajpgi.00341.2014
Calcium-sensing receptor stimulates Cl(-)- and SCFA-dependent but inhibits cAMP-dependent HCO3(-) secretion in colon
Abstract
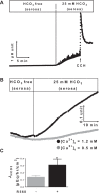
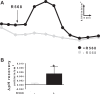
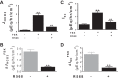
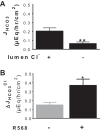
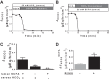
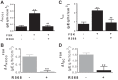
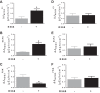
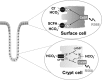
Colonic bicarbonate (HCO3(-)) secretion is a well-established physiological process that is closely linked to overall fluid and electrolyte movement in the mammalian colon. These present studies show that extracellular calcium-sensing receptor (CaSR), a fundamental mechanism for sensing and regulating ionic and nutrient compositions of extracellular milieu in the small and large intestine, regulates HCO3(-) secretion. Basal and induced HCO3(-) secretory responses to CaSR agonists were determined by pH stat techniques used in conjunction with short-circuit current measurements in mucosa from rat distal colon mounted in Ussing chambers. R568, a specific CaSR activator, stimulated lumen Cl(-)- and short-chain fatty acid (SCFA)-dependent HCO3(-) secretion but inhibited cyclic nucleotide-activated HCO3(-) secretion. Consequently, at physiological conditions (either at basal or during lumen acid challenge) when electroneutral Cl(-)/HCO3(-) and SCFA/HCO3(-) exchangers dominate, CaSR stimulates HCO3(-) secretion; in contrast, in experimental conditions that stimulate fluid and HCO3(-) secretion, e.g., when forskolin activates electrogenic cystic fibrosis transmembrane conductance regulator-mediated HCO3(-) conductance, CaSR activation inhibits HCO3(-) secretion. Corresponding changes in JHCO3 (μeq·h(-1)·cm(-2), absence vs. presence of R568) were 0.18 ± 0.03 vs. 0.31 ± 0.08 under basal nonstimulated conditions and 1.85 ± 0.23 vs. 0.45 ± 0.06 under forskolin-stimulated conditions. Similarly, activation of CaSR by R568 stimulated Cl(-)- and SCFA-dependent HCO3(-) secretion and inhibited cAMP-dependent HCO3(-) secretion in colon mucosa of wild-type mice; such effects were abolished in CaSR-null mice. These results suggest a new paradigm for regulation of intestinal ion transport in which HCO3(-) secretion may be fine-tuned by CaSR in accordance with nutrient availability and state of digestion and absorption. The ability of CaSR agonists to inhibit secretagogue-induced intestinal HCO3(-) secretion suggests that modulation of CaSR activity may provide a new therapeutic approach to correct HCO3(-) deficit and metabolic acidosis, a primary cause of morbidity and mortality in acute infectious diarrheal illnesses.
Keywords: bicarbonate secretion; calcium-sensing receptor; diarrhea; short-chain fatty acid.
Copyright © 2015 the American Physiological Society.
Figures
Similar articles
-
Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system.Am J Physiol Gastrointest Liver Physiol. 2012 Jul;303(1):G60-70. doi: 10.1152/ajpgi.00425.2011. Epub 2012 Apr 19. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22517767 Free PMC article.
-
Molecular mechanisms of calcium-sensing receptor-mediated calcium signaling in the modulation of epithelial ion transport and bicarbonate secretion.J Biol Chem. 2014 Dec 12;289(50):34642-53. doi: 10.1074/jbc.M114.592774. Epub 2014 Oct 20. J Biol Chem. 2014. PMID: 25331955 Free PMC article.
-
A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca(2+)-dependent HCO3- secretion.J Physiol. 1997 Dec 1;505 ( Pt 2)(Pt 2):411-23. doi: 10.1111/j.1469-7793.1997.411bb.x. J Physiol. 1997. PMID: 9423183 Free PMC article.
-
Bicarbonate secretion: a neglected aspect of colonic ion transport.J Clin Gastroenterol. 2005 Apr;39(4 Suppl 2):S53-8. doi: 10.1097/01.mcg.0000155521.81382.3a. J Clin Gastroenterol. 2005. PMID: 15758660 Review.
-
CaSR function in the intestine: Hormone secretion, electrolyte absorption and secretion, paracrine non-canonical Wnt signaling and colonic crypt cell proliferation.Best Pract Res Clin Endocrinol Metab. 2013 Jun;27(3):385-402. doi: 10.1016/j.beem.2013.05.005. Epub 2013 Jun 21. Best Pract Res Clin Endocrinol Metab. 2013. PMID: 23856267 Review.
Cited by
-
Particulate matter 10 exposure affects intestinal functionality in both inflamed 2D intestinal epithelial cell and 3D intestinal organoid models.Front Immunol. 2023 Jun 26;14:1168064. doi: 10.3389/fimmu.2023.1168064. eCollection 2023. Front Immunol. 2023. PMID: 37435069 Free PMC article.
-
Molecular regulation of calcium-sensing receptor (CaSR)-mediated signaling.Chronic Dis Transl Med. 2024 Apr 29;10(3):167-194. doi: 10.1002/cdt3.123. eCollection 2024 Sep. Chronic Dis Transl Med. 2024. PMID: 39027195 Free PMC article. Review.
-
Nutritional and Pharmacological Targeting of the Calcium-Sensing Receptor Influences Chemically Induced Colitis in Mice.Nutrients. 2019 Dec 16;11(12):3072. doi: 10.3390/nu11123072. Nutrients. 2019. PMID: 31888253 Free PMC article.
-
Calcium-sensing receptor regulates intestinal dipeptide absorption via Ca2+ signaling and IKCa activation.Physiol Rep. 2020 Jan;8(1):e14337. doi: 10.14814/phy2.14337. Physiol Rep. 2020. PMID: 31960592 Free PMC article.
-
Calcimimetic acts on enteric neuronal CaSR to reverse cholera toxin-induced intestinal electrolyte secretion.Sci Rep. 2018 May 18;8(1):7851. doi: 10.1038/s41598-018-26171-4. Sci Rep. 2018. PMID: 29777154 Free PMC article.
References
-
- Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol 288: C1–C19, 2005. - PubMed
-
- Binder HJ, Rajendran V, Sadasivan V, Geibel JP. Bicarbonate secretion: a neglected aspect of colonic ion transport. J Clin Gastroenterol 39: S53–S58, 2005. - PubMed
-
- Black R, Cousens S, Johnson H, Lawn J, Rudan I, Bassani D, Jha P, Campbell H, Walker C, Cibulskis R, Eisele T, Liu L, Mathers C. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375: 1969–1987, 2008. - PubMed
-
- Bovee-Oudenhoven IM, Lettink-Wissink ML, Van Doesburg W, Witteman BJ, Van Der Meer R. Diarrhea caused by enterotoxigenic Escherichia coli infection of humans is inhibited by dietary calcium. Gastroenterology 125: 469–476, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

