The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation
- PMID: 25791526
- PMCID: PMC4419282
- DOI: 10.1016/j.ajpath.2015.01.020
The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation
Abstract
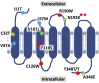
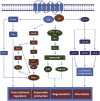
Neutrophil migration to sites of inflammation and the subsequent execution of multiple functions are designed to contain and kill invading pathogens. These highly regulated and orchestrated processes are controlled by interactions between numerous receptors and their cognate ligands. Unraveling and identifying those that are central to inflammatory processes may represent novel therapeutic targets for the treatment of neutrophil-dominant inflammatory disorders in which dysregulated neutrophil recruitment, function, and elimination serve to potentiate rather than resolve an initial inflammatory insult. The first G protein-coupled receptor to be described on human neutrophils, formyl peptide receptor 1 (FPR1), is one such receptor that plays a significant role in the execution of these functions through multiple intracellular signaling pathways. Recent work has highlighted important observations with regard to both receptor function and the importance and functional relevance of FPR1 in the pathogenesis of a range of both sterile and infective inflammatory conditions. In this review, we explore the multiple components of neutrophil migration and function in both health and disease, with a focus on the role of FPR1 in these processes. The current understanding of FPR1 structure, function, and signaling is examined, alongside discussion of the potential importance of FPR1 in inflammatory diseases suggesting that FPR1 is a key regulator of the inflammatory environment.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
The potential impacts of formyl peptide receptor 1 in inflammatory diseases.Front Biosci (Elite Ed). 2016 Jun 1;8(3):436-49. doi: 10.2741/E778. Front Biosci (Elite Ed). 2016. PMID: 27100350 Review.
-
Novel role for endogenous mitochondrial formylated peptide-driven formyl peptide receptor 1 signalling in acute respiratory distress syndrome.Thorax. 2017 Oct;72(10):928-936. doi: 10.1136/thoraxjnl-2017-210030. Epub 2017 May 3. Thorax. 2017. PMID: 28469031 Free PMC article.
-
Basic characteristics of the neutrophil receptors that recognize formylated peptides, a danger-associated molecular pattern generated by bacteria and mitochondria.Biochem Pharmacol. 2016 Aug 15;114:22-39. doi: 10.1016/j.bcp.2016.04.014. Epub 2016 Apr 27. Biochem Pharmacol. 2016. PMID: 27131862 Review.
-
Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1.J Immunol. 2013 Jun 15;190(12):6511-9. doi: 10.4049/jimmunol.1202215. Epub 2013 May 13. J Immunol. 2013. PMID: 23670191
-
Honokiol suppresses formyl peptide-induced human neutrophil activation by blocking formyl peptide receptor 1.Sci Rep. 2017 Jul 27;7(1):6718. doi: 10.1038/s41598-017-07131-w. Sci Rep. 2017. PMID: 28751674 Free PMC article.
Cited by
-
Bone Marrow-Derived IL-1Ra Increases TNF Levels Poststroke.Cells. 2021 Apr 20;10(4):956. doi: 10.3390/cells10040956. Cells. 2021. PMID: 33924148 Free PMC article.
-
The principles of directed cell migration.Nat Rev Mol Cell Biol. 2021 Aug;22(8):529-547. doi: 10.1038/s41580-021-00366-6. Epub 2021 May 14. Nat Rev Mol Cell Biol. 2021. PMID: 33990789 Free PMC article. Review.
-
Mechanisms of Virus-Induced Airway Immunity Dysfunction in the Pathogenesis of COPD Disease, Progression, and Exacerbation.Front Immunol. 2020 Jun 16;11:1205. doi: 10.3389/fimmu.2020.01205. eCollection 2020. Front Immunol. 2020. PMID: 32655557 Free PMC article. Review.
-
Identification of FPR3 as a Unique Biomarker for Targeted Therapy in the Immune Microenvironment of Breast Cancer.Front Pharmacol. 2021 Feb 11;11:593247. doi: 10.3389/fphar.2020.593247. eCollection 2020. Front Pharmacol. 2021. PMID: 33679387 Free PMC article.
-
Regulation of inflammation by lipid mediators in oral diseases.Oral Dis. 2017 Jul;23(5):576-597. doi: 10.1111/odi.12544. Epub 2016 Aug 4. Oral Dis. 2017. PMID: 27426637 Free PMC article. Review.
References
-
- Cavaillon J.M. The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J Leukoc Biol. 2011;90:413–424. - PubMed
-
- Jaillon S., Galdiero M.R., Del Prete D., Cassatella M.A., Garlanda C., Mantovani A. Neutrophils in innate and adaptive immunity. Semin Immunopathol. 2013;35:377–394. - PubMed
-
- Nourshargh S., Hordijk P.L., Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. - PubMed
-
- Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. - PubMed
-
- Boulay F., Tardif M., Brouchon L., Vignais P. Synthesis and use of a novel N-formyl peptide derivative to isolate a human N-formyl peptide receptor cDNA. Biochem Biophys Res Commun. 1990;168:1103–1109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

