Evaluation of optimal electrode configurations for epidural spinal cord stimulation in cervical spinal cord injured rats
- PMID: 25791014
- PMCID: PMC4465788
- DOI: 10.1016/j.jneumeth.2015.03.012
Evaluation of optimal electrode configurations for epidural spinal cord stimulation in cervical spinal cord injured rats
Erratum in
- J Neurosci Methods. 2015 Oct 30;254:102-3
Abstract
Background: Epidural spinal cord stimulation is a promising technique for modulating the level of excitability and reactivation of dormant spinal neuronal circuits after spinal cord injury (SCI). We examined the ability of chronically implanted epidural stimulation electrodes within the cervical spinal cord to (1) directly elicit spinal motor evoked potentials (sMEPs) in forelimb muscles and (2) determine whether these sMEPs can serve as a biomarker of forelimb motor function after SCI.
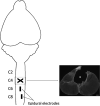
New method: We implanted EMG electrodes in forelimb muscles and epidural stimulation electrodes at C6 and C8 in adult rats. After recovering from a dorsal funiculi crush (C4), rats were tested with different stimulation configurations and current intensities to elicit sMEPs and determined forelimb grip strength.
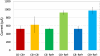
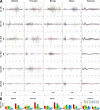
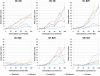
Results: sMEPs were evoked in all muscles tested and their characteristics were dependent on electrode configurations and current intensities. C6(-) stimulation elicited more robust sMEPs than stimulation at C8(-). Stimulating C6 and C8 simultaneously produced better muscle recruitment and higher grip strengths than stimulation at one site.
Comparison with existing method(s): Classical method to select the most optimal stimulation configuration is to empirically test each combination individually for every subject and relate to functional improvements. This approach is impractical, requiring extensively long experimental time to determine the more effective stimulation parameters. Our proposed method is fast and physiologically sound.
Conclusions: Results suggest that sMEPs from forelimb muscles can be useful biomarkers for identifying optimal parameters for epidural stimulation of the cervical spinal cord after SCI.
Keywords: Cervical spinal cord injury; Dorsal funiculi crush; Epidural stimulation; Motor evoked potentials.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Electrical neuromodulation of the cervical spinal cord facilitates forelimb skilled function recovery in spinal cord injured rats.Exp Neurol. 2017 May;291:141-150. doi: 10.1016/j.expneurol.2017.02.006. Epub 2017 Feb 10. Exp Neurol. 2017. PMID: 28192079 Free PMC article.
-
Combined motor cortex and spinal cord neuromodulation promotes corticospinal system functional and structural plasticity and motor function after injury.Exp Neurol. 2016 Mar;277:46-57. doi: 10.1016/j.expneurol.2015.12.008. Epub 2015 Dec 18. Exp Neurol. 2016. PMID: 26708732 Free PMC article.
-
Therapeutic intraspinal microstimulation improves forelimb function after cervical contusion injury.J Neural Eng. 2013 Aug;10(4):044001. doi: 10.1088/1741-2560/10/4/044001. Epub 2013 May 28. J Neural Eng. 2013. PMID: 23715242 Free PMC article.
-
Emergence of Epidural Electrical Stimulation to Facilitate Sensorimotor Network Functionality After Spinal Cord Injury.Neuromodulation. 2019 Apr;22(3):244-252. doi: 10.1111/ner.12938. Epub 2019 Mar 6. Neuromodulation. 2019. PMID: 30840354 Review.
-
Electrical epidural stimulation of the cervical spinal cord: implications for spinal respiratory neuroplasticity after spinal cord injury.J Neurophysiol. 2021 Aug 1;126(2):607-626. doi: 10.1152/jn.00625.2020. Epub 2021 Jul 7. J Neurophysiol. 2021. PMID: 34232771 Free PMC article. Review.
Cited by
-
When Spinal Neuromodulation Meets Sensorimotor Rehabilitation: Lessons Learned From Animal Models to Regain Manual Dexterity After a Spinal Cord Injury.Front Rehabil Sci. 2021 Dec 7;2:755963. doi: 10.3389/fresc.2021.755963. eCollection 2021. Front Rehabil Sci. 2021. PMID: 36188826 Free PMC article. Review.
-
Effective robotic assistive pattern of treadmill training for spinal cord injury in a rat model.Exp Ther Med. 2018 Apr;15(4):3283-3294. doi: 10.3892/etm.2018.5822. Epub 2018 Jan 31. Exp Ther Med. 2018. PMID: 29545846 Free PMC article.
-
Engaging Cervical Spinal Cord Networks to Reenable Volitional Control of Hand Function in Tetraplegic Patients.Neurorehabil Neural Repair. 2016 Nov;30(10):951-962. doi: 10.1177/1545968316644344. Epub 2016 May 18. Neurorehabil Neural Repair. 2016. PMID: 27198185 Free PMC article.
-
Development of a battery-free ultrasonically powered functional electrical stimulator for movement restoration after paralyzing spinal cord injury.J Neuroeng Rehabil. 2019 Mar 8;16(1):36. doi: 10.1186/s12984-019-0501-4. J Neuroeng Rehabil. 2019. PMID: 30850027 Free PMC article.
-
Generation of locomotor‑like activity using monopolar intraspinal electrical microstimulation in rats.Exp Ther Med. 2023 Oct 18;26(6):560. doi: 10.3892/etm.2023.12259. eCollection 2023 Dec. Exp Ther Med. 2023. PMID: 37941590 Free PMC article.
References
-
- Alam M, He J. Lower-limb neuroprostheses: restoring walking after spinal cord injury. In: Naik GR, Guo Y, editors. Emerging theory and practice in neuroprosthetics. IGI Global; Hershey, PA, USA: 2014. pp. 153–80.
-
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

