Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling
- PMID: 25789082
- PMCID: PMC4348608
- DOI: 10.1155/2015/867293
Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling
Abstract
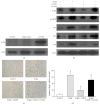
Increasing evidences indicate that reactive oxygen species are the main factor promoting stem cell aging. Recent studies have demonstrated that coenzyme Q10 (CoQ10) plays a positive role in organ and cellular aging. However, the potential for CoQ10 to protect stem cell aging has not been fully evaluated, and the mechanisms of cell senescence inhibited by CoQ10 are still poorly understood. Our previous study had indicated that D-galactose (D-gal) can remarkably induce mesenchymal stem cell (MSC) aging through promoting intracellular ROS generation. In this study, we showed that CoQ10 could significantly inhibit MSC aging induced by D-gal. Moreover, in the CoQ10 group, the expression of p-Akt and p-mTOR was clearly reduced compared with that in the D-gal group. However, after Akt activating by CA-Akt plasmid, the senescence-cell number in the CoQ10 group was significantly higher than that in the control group. These results indicated that CoQ10 could inhibit D-gal-induced MSC aging through the Akt/mTOR signaling.
Figures
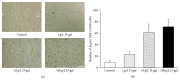

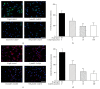
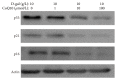
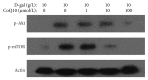
Similar articles
-
Cholesterol Retards Senescence in Bone Marrow Mesenchymal Stem Cells by Modulating Autophagy and ROS/p53/p21Cip1/Waf1 Pathway.Oxid Med Cell Longev. 2016;2016:7524308. doi: 10.1155/2016/7524308. Epub 2016 Sep 15. Oxid Med Cell Longev. 2016. PMID: 27703600 Free PMC article.
-
High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling.Mol Med Rep. 2017 Aug;16(2):1685-1690. doi: 10.3892/mmr.2017.6832. Epub 2017 Jun 21. Mol Med Rep. 2017. PMID: 28656269 Free PMC article.
-
Hydrogen alleviates cellular senescence via regulation of ROS/p53/p21 pathway in bone marrow-derived mesenchymal stem cells in vivo.Biomed Pharmacother. 2018 Oct;106:1126-1134. doi: 10.1016/j.biopha.2018.07.020. Epub 2018 Jul 17. Biomed Pharmacother. 2018. PMID: 30119179
-
Aberrant mTOR activation in senescence and aging: A mitochondrial stress response?Exp Gerontol. 2015 Aug;68:66-70. doi: 10.1016/j.exger.2014.11.004. Epub 2014 Nov 6. Exp Gerontol. 2015. PMID: 25449851 Free PMC article. Review.
-
[Reactive oxygen species and bone marrow hematopoietic stem cell senescence].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012 Dec;20(6):1518-21. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012. PMID: 23257465 Review. Chinese.
Cited by
-
Biomolecules resveratrol + coenzyme Q10 recover the cell state of human mesenchymal stem cells after 1-methyl-4-phenylpyridinium-induced damage and improve proliferation and neural differentiation.Front Neurosci. 2022 Aug 31;16:929590. doi: 10.3389/fnins.2022.929590. eCollection 2022. Front Neurosci. 2022. PMID: 36117620 Free PMC article.
-
In search of elixir: Pharmacological agents against stem cell senescence.Iran J Basic Med Sci. 2021 Jul;24(7):868-880. doi: 10.22038/ijbms.2021.51917.11773. Iran J Basic Med Sci. 2021. PMID: 34712416 Free PMC article. Review.
-
Coenzyme Q10 Regulation of Apoptosis and Oxidative Stress in H2O2 Induced BMSC Death by Modulating the Nrf-2/NQO-1 Signaling Pathway and Its Application in a Model of Spinal Cord Injury.Oxid Med Cell Longev. 2019 Dec 12;2019:6493081. doi: 10.1155/2019/6493081. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31915512 Free PMC article.
-
Targeting Mitochondria to Control Ageing and Senescence.Pharmaceutics. 2023 Jan 20;15(2):352. doi: 10.3390/pharmaceutics15020352. Pharmaceutics. 2023. PMID: 36839673 Free PMC article. Review.
-
Mechanism of Bazi Bushen capsule in delaying the senescence of mesenchymal stem cells based on network pharmacology and experimental validation.Heliyon. 2024 Mar 9;10(6):e27646. doi: 10.1016/j.heliyon.2024.e27646. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38509951 Free PMC article.
References
-
- Ishikane S., Hosoda H., Yamahara K., et al. Allogeneic transplantation of fetal membrane-derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation. 2013;96(8):697–706. doi: 10.1097/TP.0b013e31829f753d. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

