Cdk5 controls IL-2 gene expression via repression of the mSin3a-HDAC complex
- PMID: 25785643
- PMCID: PMC4614394
- DOI: 10.4161/15384101.2014.987621
Cdk5 controls IL-2 gene expression via repression of the mSin3a-HDAC complex
Abstract
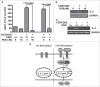
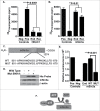
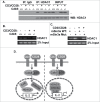
Cyclin-dependent kinase 5 (Cdk5) is a unique member of a family of serine/threonine cyclin-dependent protein kinases. We previously demonstrated disruption of Cdk5 gene expression in mice impairs T-cell function and ameliorates T-cell-mediated neuroinflammation. Here, we show Cdk5 modulates gene expression during T-cell activation by impairing the repression of gene transcription by histone deacetylase 1 (HDAC1) through specific phosphorylation of the mSin3a protein at serine residue 861. Disruption of Cdk5 activity in T-cells enhances HDAC activity and binding of the HDAC1/mSin3a complex to the IL-2 promoter, leading to suppression of IL-2 gene expression. These data point to essential roles for Cdk5 in regulating gene expression in T-cells and transcriptional regulation by the co-repressor mSin3a.
Figures


Similar articles
-
Cyclin-dependent kinase 5 represses Foxp3 gene expression and Treg development through specific phosphorylation of Stat3 at Serine 727.Mol Immunol. 2015 Oct;67(2 Pt B):317-24. doi: 10.1016/j.molimm.2015.06.015. Epub 2015 Jul 19. Mol Immunol. 2015. PMID: 26198700 Free PMC article.
-
The transcription repressor, ZEB1, cooperates with CtBP2 and HDAC1 to suppress IL-2 gene activation in T cells.Int Immunol. 2009 Mar;21(3):227-35. doi: 10.1093/intimm/dxn143. Epub 2009 Jan 30. Int Immunol. 2009. PMID: 19181930
-
Association of the mSin3A-histone deacetylase 1/2 corepressor complex with the mouse steroidogenic acute regulatory protein gene.Mol Endocrinol. 2006 Jan;20(1):100-13. doi: 10.1210/me.2004-0495. Epub 2005 Aug 18. Mol Endocrinol. 2006. PMID: 16109738
-
Cdk5/p35 phosphorylates mSds3 and regulates mSds3-mediated repression of transcription.J Biol Chem. 2004 Dec 24;279(52):54438-44. doi: 10.1074/jbc.M411002200. Epub 2004 Oct 15. J Biol Chem. 2004. PMID: 15489224
-
Smad3-mSin3A-HDAC1 Complex is Required for TGF-β1-Induced Transcriptional Inhibition of PPARγ in Mouse Cardiac Fibroblasts.Cell Physiol Biochem. 2016;40(5):908-920. doi: 10.1159/000453149. Epub 2016 Dec 7. Cell Physiol Biochem. 2016. PMID: 27941310
Cited by
-
The transcriptional regulator Sin3A balances IL-17A and Foxp3 expression in primary CD4 T cells.EMBO Rep. 2023 May 4;24(5):e55326. doi: 10.15252/embr.202255326. Epub 2023 Mar 16. EMBO Rep. 2023. PMID: 36929576 Free PMC article.
-
p35 is a Crucial Player in NK-cell Cytotoxicity and TGFβ-mediated NK-cell Dysfunction.Cancer Res Commun. 2023 May 5;3(5):793-806. doi: 10.1158/2767-9764.CRC-22-0497. eCollection 2023 May. Cancer Res Commun. 2023. PMID: 37377891 Free PMC article.
-
Purification and identification of a polysaccharide from medicinal mushroom Amauroderma rude with immunomodulatory activity and inhibitory effect on tumor growth.Oncotarget. 2015 Jul 10;6(19):17777-91. doi: 10.18632/oncotarget.4397. Oncotarget. 2015. PMID: 26219260 Free PMC article.
-
Estrogen receptor alpha and NFATc1 bind to a bone mineral density-associated SNP to repress WNT5B in osteoblasts.Am J Hum Genet. 2022 Jan 6;109(1):97-115. doi: 10.1016/j.ajhg.2021.11.018. Epub 2021 Dec 13. Am J Hum Genet. 2022. PMID: 34906330 Free PMC article.
-
Cyclin-dependent kinase 5 negatively regulates antiviral immune response by disrupting myeloid differentiation primary response protein 88 self-association.Virulence. 2023 Dec;14(1):2223394. doi: 10.1080/21505594.2023.2223394. Virulence. 2023. PMID: 37332205 Free PMC article.
References
-
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol 2001; 2:749-59; PMID:11584302; http://dx.doi.org/10.1038/35096019 - DOI - PubMed
-
- Arif A. Extraneuronal activities and regulatory mechanisms of the atypical cyclin-dependent kinase Cdk5. Biochem Pharmacol 2012; 84:985-93; PMID:22795893; http://dx.doi.org/10.1016/j.bcp.2012.06.027 - DOI - PubMed
-
- Jessberger S, Gage FH, Eisch AJ, Lagace DC. Making a neuron: Cdk5 in embryonic and adult neurogenesis. Trends Neurosci 2009; 32:575-82; PMID:19782409; http://dx.doi.org/10.1016/j.tins.2009.07.002 - DOI - PMC - PubMed
-
- Pareek TK, Lam E, Zheng X, Askew D, Kulkarni AB, Chance MR, Huang AY, Cooke KR, Letterio JJ. Cyclin-dependent kinase 5 activity is required for T cell activation and induction of experimental autoimmune encephalomyelitis. J Exp Med 2010; 207:2507-19; PMID:20937706; http://dx.doi.org/10.1084/jem.20100876 - DOI - PMC - PubMed
-
- Nohara A, Okada S, Ohshima K, Pessin JE, Mori M. Cyclin-dependent kinase-5 is a key molecule in tumor necrosis factor-alpha-induced insulin resistance. J Biol Chem 2011; 286:33457-65; PMID:21813649; http://dx.doi.org/10.1074/jbc.M111.231431 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
